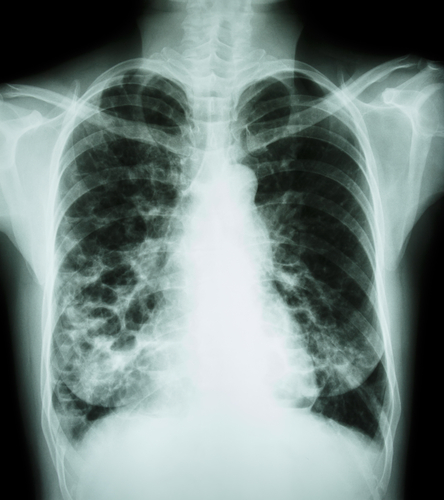
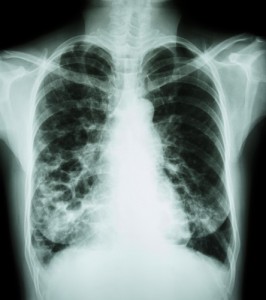
 Bayer HealthCare’s Ciprofloxacin Dry Powder for Inhalation, a treatment for non-cystic fibrosis bronchiectasis (NCFB), was recently granted orphan drug designation by the U.S Food and Drug Administration’s (FDA) Office of Orphan Products Development.
Bayer HealthCare’s Ciprofloxacin Dry Powder for Inhalation, a treatment for non-cystic fibrosis bronchiectasis (NCFB), was recently granted orphan drug designation by the U.S Food and Drug Administration’s (FDA) Office of Orphan Products Development.
Being a rare disease (an orphan disease) that affects no more than 110,000 U.S people, the orphan drug designation is meant to encourage research and development into effective drugs may not be appealing to pharmaceutical companies, because their investment would probably never be recovered with such a low rate of potential patient-customers.
The determination of an “orphan drug” may offer drug developers some tax advantages, including tax credits for qualified clinical testing.
Based on the recent Drug Orphan Act, companies like Bayer HealthCare could start developing orphan drugs like Ciprofloxacin Dry Powder for Inhalation (Ciprofloxacin DPI) for long-term intermittent treatment for decreasing the frequency of acute exacerbations in NCFB individuals with respiratory bacterial infections.
[adrotate group=”6″]
Non-cystic fibrosis is a condition in which the tubes that transport air to the lungs become scarred and twisted, which leads frequently to lung infections and causes morbidity and mortality.
In the U.S., there is currently no approved drug treatment, except for therapies for established chest infections (which are typically treated using antibiotics).
According to the British Lung Foundation the regular treatment to be adopted isn’t a drug but a “regular chest physiotherapy to prevent the build-up of mucus and the development of chest infections.”