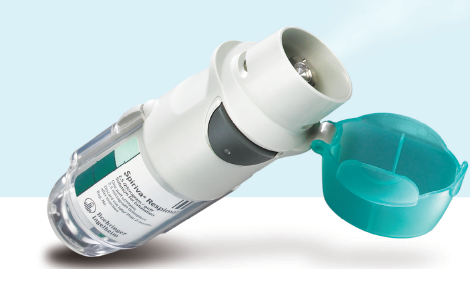
Boehringer Ingelheim Pharmaceuticals, Inc. has just announced the nationwide retail availability of prescription SPIRIVA®RESPIMAT® (tiotropium bromide) Inhalation Spray, which was recently granted US Food and Drug Administration approval as a long-term, once-daily maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), such as chronic bronchitis and emphysema. The company also noted that leading COPD maintenance therapy, Spiriva® HandiHaler® (tiotropium bromide inhalation powder), will remain available despite the newly released inhalation spray product.
Boehringer Ingelheim Pharmaceuticals, Inc. has just announced the nationwide retail availability of prescription SPIRIVA®RESPIMAT® (tiotropium bromide) Inhalation Spray, which was recently granted US Food and Drug Administration approval as a long-term, once-daily maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), such as chronic bronchitis and emphysema. The company also noted that leading COPD maintenance therapy, Spiriva® HandiHaler® (tiotropium bromide inhalation powder), will remain available despite the newly released inhalation spray product.
“For 10 years, SPIRIVA HandiHaler has been a trusted maintenance therapy for patients with COPD, and Boehringer Ingelheim is proud to now offer another inhaler choice, SPIRIVA RESPIMAT,” said Danny McBryan, MD, vice president, Clinical Development & Medical Affairs, Respiratory, Boehringer Ingelheim Pharmaceuticals, Inc. “SPIRIVA RESPIMAT represents Boehringer Ingelheim’s continuing commitment to the COPD community to provide new treatment options.”
Spiriva Respimat was created to offer patients a medication delivery system that uses slow-moving mist, eliminating the need for inspiratory effort on the patient’s part. While adequate medication delivery is affected by a number of variables, including device usage and quality of inspiration, Boehringer is confident the Respimat system will be another trusted method to control COPD.
[adrotate group=”3″]
The Company will be offering eligible patients a savings card that will allow them to purchase the new Spiriva Respimat for a low price of $10, depending on their insurance provider. Those interested to avail of the savings card can visit www.spiriva.com. Once obtained, the card can be activated at most pharmacies all over the country. To learn more about Boehringer Ingelheim’s patient assistance service, BI Cares, visit www.BIPatientAssistance.com.
Spiriva Respimat is only the 3rd FDA-approved product compatible with the Respimat system, which is the only inhaler sold in the United States that utilizes mechanical energy to produce a slow-moving mist to deliver the medication and features a dose indicator to help the user keep track of how much of the medication is left.
Boehringer Ingelheim operates globally with 140 affiliates and more than 47,000 employees. The company’s key assets of interest are: respiratory diseases, cardiovascular diseases, Parkinson’s disease, HIV, thromboembolic diseases, cerebrovascular diseases, oncology, diabetes and hepatitis.