Researchers found that because expression levels of PD-L1 protein vary in patients’ tumors from non–small cell lung cancer (NSCLC), additional studies are required to develop predictive biomarkers for NSCLC. The study entitled “Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non–Small-Cell Lung Cancer,” was published in the journal JAMA Oncology.
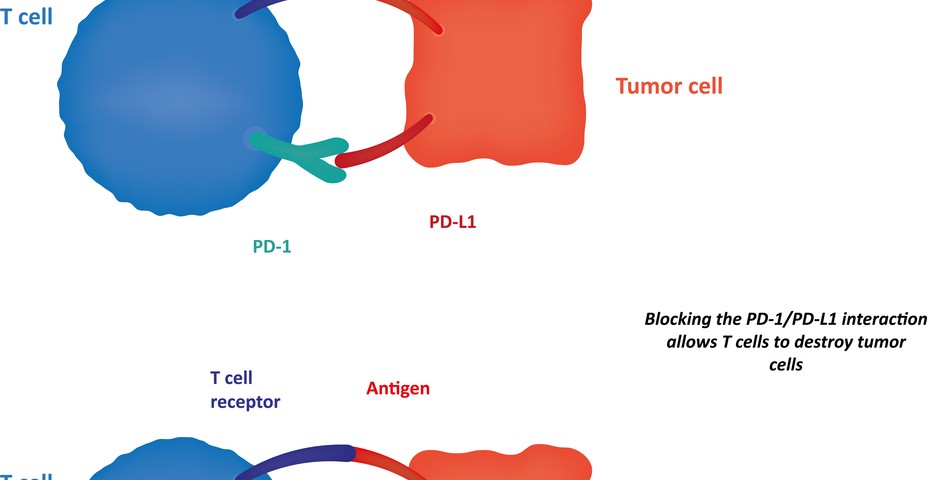
Early-phase clinical trials with antibodies targeting PD-1 (programmed cell death protein 1) and PD-L1 (programmed cell death 1 ligand 1) showed an effective and durable response in patients with NSCLC. PD-1 is an immune checkpoint that plays a key role in inhibiting immune system responses; therefore its expression is commonly upregulated in many cancers, including NSCLC.
Researchers investigated how PD-L1 protein expression is distributed in 49 patients with NSCLC. To this end, they used two different methods, either immunohistochemistry (IHC) or quantitative immunofluorescence (QIF), and they assessed PD-L1 distribution with two antibodies, E1L3N and SP142, 2 rabbit monoclonal antibodies (antibodies that recognize and have affinity for the same antigen, i.e., any substance that causes an immune system to produce antibodies against it).
They observed heterogeneity in PD-L1 expression in NSCLC tumors for both E1L3N and SP142 monoclonal antibodies, and intra-assay heterogeneity. Moreover, the results obtained with IHC and QIF showed fair to poor concordance.
Joseph McLaughlin, MD, from the department of medical oncology at Yale University School of Medicine, and colleagues noted in a press release, “Future studies measuring PD-L1 protein quantitatively in patients treated with anti–PD-1 and anti–PD-L1 therapies may better address the prognostic and/or predictive value of these biomarkers. Determination of the optimal assay, PD-L1 antibody, and the best cut-point for PD-L1 positivity, will require further rigorous studies including tissues with known response to anti–PD-1 and anti–PD-L1 therapies.”
These findings were also the subject of an editorial by Feriyl Bhaijee, MD, from AmeriPath Indiana in Indianapolis and Robert A. Anders, MD, PhD, from the department of pathology at Johns Hopkins University, who noted, “Identifying the subset of patients who are most likely to respond to anti–PD-L1 therapy is the first step toward individualized therapy. As a biomarker, however, PD-L1 immunohistochemistry offers poor sensitivity and reproducibility, and as a result, these patients may be unfairly excluded from clinical trials. To offer this potentially life-saving personalized immune-based therapy to as many patients as possible, we need to develop a multifaceted predictive biomarker system that integrates checkpoint inhibitors such as PD-L1, tumor mutations, and inflammatory cells.”