Pulmonx announced that patient enrollment is complete for its pivotal investigational device exemption (IDE) study of the Zephyr Endobronchial Valve (EBV) for the treatment of severe emphysema.
Results from the randomized, multi-center LIBERATE clinical trial (NCT01796392) are expected to support approval of the device by the U.S. Food and Drug Administration (FDA).
The LIBERATE trial enrolled 190 patients in 24 centers to assess Zephyr EBVs’ safety and effectiveness versus optimal medical management, with patients randomized at 2:1 ratio (Zephyr EBV vs. control group).
The trial’s primary objective is to evaluate lung function, measured by forced expiratory volume in one second (FEV1) at one year. Secondary outcomes are volume reduction of the treated lobe of the lung, improvement in exercise capacity (assessed through the six-minute walk distance, or 6MWD, test), and changes in quality of life assessed through the St. George’s Respiratory Questionnaire (SGRQ).
Severe emphysema is a form of chronic obstructive pulmonary disease (COPD) that refers to an abnormal permanent enlargement of the distal airspace and the terminal bronchioles, along with the loss of elasticity of alveolar walls and the supporting structures feeding the alveoli.
Emphysema eventually reduces a patient’s capacity to inhale and exhale air, and reduces the overall surface area for gas exchange.
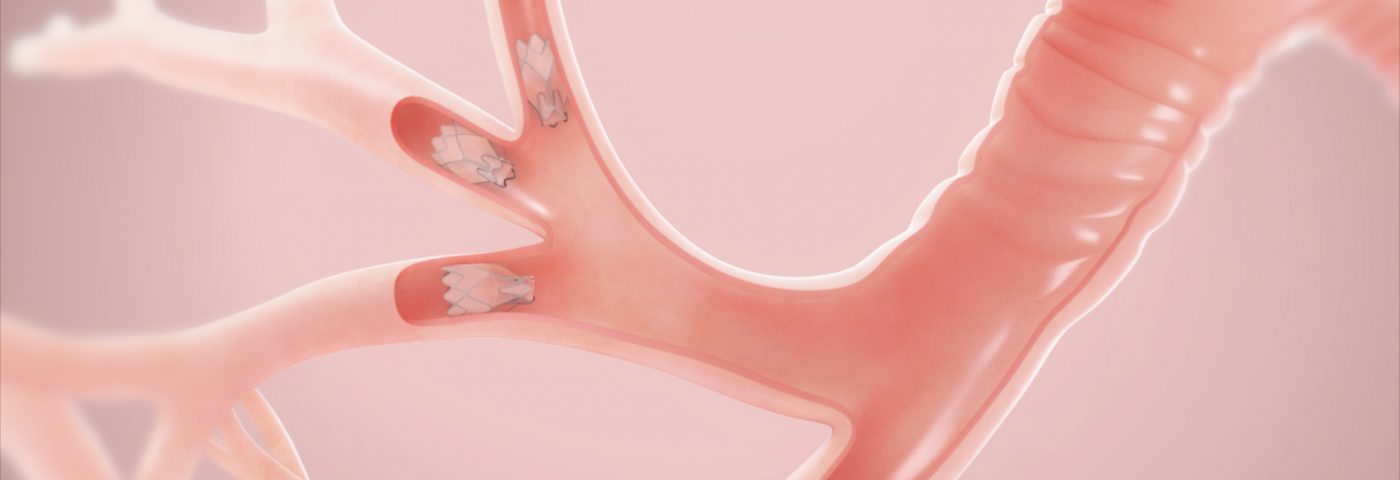
Zephyr EBVs are small, minimally-invasive, one-way valves that are placed in selected airways of the lungs to occlude damaged regions and reduce lung hyperinflation. This process results in a more efficient functioning of the remaining healthier lung regions, enabling improved breathing capacity and a higher quality of life.
Studies like “Predicting Lung Volume Reduction after Endobronchial Valve Therapy Is Maximized Using a Combination of Diagnostic Tools,” published in the scientific journal Respiration, have shown that over 50% of severe emphysema patients have no collateral ventilation in one or more target lobes of the lung, which demonstrates a large potential market for Zephyr EBV.
“The Zephyr EBV is the most studied endoscopic lung volume reduction device globally and is considered first-line therapy by leading physicians outside of the U.S.,” Pulmonx CEO Glen French said in a press release. “Completing enrollment in this important trial moves us one step closer to making this proven treatment available to U.S. patients who have few options today.”
Three previous randomized clinical trials of Zephyr EBV, conducted outside the United States – BeLieVeR-HIFi (ISRCTN04761234), STELVIO (NTR2876), and IMPACT (NCT02025205) – have shown that the device was capable of significantly improving emphysema patients’ lung function, exercise tolerance, and quality of life in cases where there was no collateral ventilation, as assessed by the Chartis System.
Other published data (a full list is available in the press release) has also indicated that Zephyr EBV leads to sustained patient benefits up to five years, and potential survival benefits at five and 10 years after treatment, indicating a potential deceleration of disease progression. Over the past decade, more than 40,000 Zephyr EBVs were implanted globally in over 12,000 patients.
Here is an informational video of the full Zephyr EBV procedure:

That is what I’ve hope for ,sure hope it’s available here in The USA soon ,this sounds like what we need ,
Geoff Sykes
I am resident in Adelaide South Australia where I undertook this procedure in April 2015. My surgeons initially placed six (6) EBV’s into the upper lobe of my left lung; the procedure takes approximately 90 minutes from start to finish under general anesthetic.
Whilst movement initially is restrictive (to ensure that valves seat and settle effectively)pain and post operational activity associated with the procedure is not greatly affected. You will be encouraged to commence as quickly as possible your rehab program (which is essential to the efficiency of the procedure)and monitored for lung function regularly.
Patients, however need to be aware that- even though the surgery is a major advance towards improving lifestyle and general health it is not the “silver bullet” which will have you out running marathons as a result. There are and can be complications which may be difficult to foresee such as fluid build-up around the valves (creating pneumonia like symptoms)or valves shifting (the result of coughing)and causing air leakage plus other considerations which you as a patient will become aware of in due course- however I can testify that this surgery is a huge step forward in lung surgery and if suitable a far better alternative to lung transplant.
My thoughts and best wishes go with you if you are about to undergo the procedure.
How have the valves worked out for you. I’m trying to get int0 a clinical in the US but if the benefit is great enough, I’ll go to London as a private patient and pay for the procedure out of pocket. An honest and realalistic estimation of the effacy would be appreciated.
will they ever do this or any other new treatment in Ireland we get no real help at all
Hi Francis
Hopefully yes they will in time for you to reap some benefits from it – in the meantime though, don’t underestimate the benefits that can come from a simple rehab regime. Something as simple as setting aside 30 minutes each day to walk! – I try to make sure that I do 3 X 10 minute short walks each day – and at times when I am struggling those 10 minutes can be broken up into even smaller segments, but the rewards are worth it, if the weather won’t allow you outside find a simple path that you can follow inside.
Finally – not sure what stage you are in your illness (I am stage 3) according to my surgeon and the tests I am regularly undertaking but don’t let that put you off “doing things” it is really easy to convince yourself that you aren’t up to it on any day, to beat this horrible disease you have to control it and push through, don’t allow it to dictate.
Take care – I hope everything turns out well for you.
Geoff
My brother had 3 valves placed (2 in the 1st intervention and 1 year later)
The surgeon wanted to see if in his case it would work with only 2, but the progress was slow and my brother decided to ask to have the 3rd one placed.
It took another 6 months to see progress, but today he can walk for a block or two without stopping to catch his breath while before that he could not walk more than a few steps without stopping. Now he can do things at home and he can shower without having to reach for the oxygen.
We are all very happy with his progress as well as with Dr Oliveira’ s care.
glad to see it worked! How much did his FEV 1 increase? What center did the trial? Was his lung damage isolated to one lung or were both involved?
Good luck and hope improvement continues!
Is there a way to contact you, as we are looking to find a place to have this treatment done. Also talk to someone that has actually gone through the procedure. Thanks Dee
I’m stage 4 and on oxygen 24/7 would I benefit from this procedure! It sounds wonderful and is there a clinic in Texas ?
Margaret Newberry