Minneapolis based Nonin Medical, Inc., inventor of finger pulse oximetry, announced Thursday that the U.S. Food and Drug Administration (FDA) has cleared the Nonin Model 3231 OEM/eHealth finger pulse oximeter for use in the United States, a medical device that can prove useful for patients suffering from COPD.
 This oximeter plugs into a telemedicine hub or kiosk through a USB connector to measure oxygen saturation through a USB connector and measures oxygen saturation and pulse rate in pediatric to adult patients. The Model 3231 received EU certification last year.
This oximeter plugs into a telemedicine hub or kiosk through a USB connector to measure oxygen saturation through a USB connector and measures oxygen saturation and pulse rate in pediatric to adult patients. The Model 3231 received EU certification last year.
“Leading telemedicine providers such as HealthSpot and Bosch Healthcare have chosen to integrate the Nonin 3231 into their telehealth systems because they expect the 3231 to provide superior performance in the widest patient population, including challenging patients,” says Nonin Medical’s Vice President of eHealth and OEM Mark VanderWerf in a release.
“Unlike some imported oximeters, Nonin’s American-made Model 3231 is a medical device with clinically proven accuracy, not a health and wellness gadget,” Mr. VanderWerf notes. “This is important to telemedicine providers because they are measuring and guiding decisions on real patients with real diagnosed diseases such as COPD, CHF and asthma. They depend on the clinical accuracy of a real medical device.”
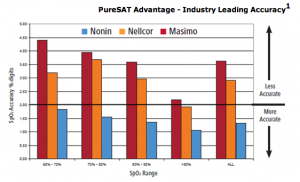
The company says its Model 3231 oximeter features accuracy advantages, including Nonin’s PureSAT Pulse Oximetry (SpO2) technology, which utilizes intelligent pulse-by-pulse filtering to provide precise oximetry measurements even in the presence of motion, dark skin tones, low perfusion, rapid SpO2 changes, and other challenging conditions. PureSAT automatically adjusts to each patient’s condition to provide fast and reliable readings that clinicians can act on.
In a controlled laboratory study of 36 subjects, researchers at the University of California San Francisco found that dark skin can result in decreased accuracy of pulse oximeters, especially in combination with low oxygen saturations. In the study, the NONIN PureSAT oximetry with clip sensor was not affected by skin pigmentation compared to competitive products tested. The NONIN PureSAT oximeter with clip sensor had excellent accuracy and the lowest bias throughout the oxygen saturation levels and for all skin pigmentations. Of particular note, the authors state “clinically important bias should be considered when monitoring patients with saturation below 80%, especially those with darkly pigmented skin”. In this most challenging environment of low saturation (SaO2 70% to 80%) and dark skin pigmentation, the bias for NONIN PureSAT oximetry with the clip sensor was minimal at -0.6 ± 1.2 and the accuracy was excellent. This is in contrast to the competitor’s results with a mean bias of 2.6 ± 2.6 and 2.6 ± 3.0 in the same subgroup.
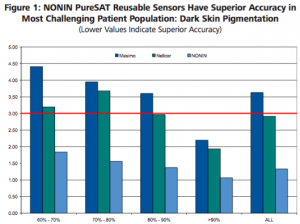
Arms
Arms calculated from Mean and SD of Bias; “Dark skin decreases the accuracy of pulse oximeters at low oxygen saturations: effects of oximeter probe type and gender,” John Feiner, et al. Anesthesia & Analgesia, December, 2007.
– Nonin’s proprietary CorrectCheck technology, which provides feedback via a digital display if the patient’s finger is not placed correctly in the device. CorrectCheck is helpful since improper finger placement may lead to incorrect readings.
– SmartPoint capture, an algorithm developed by Nonin that automatically determines when a high quality measurement is ready to be stored. This helps to ensure that each reading transmitted by the Model 3231 is accurate.
Nonin received FDA clearance in September 2013 for its wireless version of the product, the Nonin Bluetooth Smart Model 3230 finger pulse oximeter.
Nonin eHealth Pulse Oximeters
With more than 1.2 million pulse oximeters installed, Nonin is a leader among eHealth providers in integrating products for chronic disease management of conditions such as chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), sleep apnea and asthma.
Nonin Medical was in the technology vanguard with the world’s first pulse oximeter with Bluetooth wireless technology, the world’s first interoperable, fingertip pulse oximeter with Bluetooth wireless technology, and the world’s first clinical device that uses Bluetooth Smart (LE) wireless technology, with simplified pairing. The company co-founded the Continua Alliance to establish common standards, and its Onyx II 9560 is one of the very first medical devices to receive Microsoft HealthVault Certification to prove enterprise-wide interoperability. Nonin Medical is one of the few companies to be Seimens Assignio Certified. Nonin is also certified by Telus for similar eHealth solutions in Canada.
As an active participant, and Promoter Member of the Continua Alliance, Nonin is a leading company for implementation of emerging open standards, including Continua standards. The first two devices certified by the Continua Alliance were Nonin Medical products), IEEE11073, and Bluetooth’s Health Device Profile (HDP) Nonin is a leading member of the Bluetooth SIG, won its Best of CES Award for the Onyx II 9560, is actively involved in helping set healthcare data standards, and a Sustaining Circle Member and a member of the Industry Council of the American Telemedicine Association
With more than 1.2 million pulse oximeters installed, Nonin is leading eHealth providers in integrating Nonin product for chronic disease management of conditions such as chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), sleep apnea and asthma.
Click here to learn more about Nonin’s PureSAT pulse oximetry technology.
The company has applied its clinical algorithms to semi-consumer devices to achieve the clinical accuracy needed to monitor more than just health and wellness patients, but patients with diagnosed diseases like COPD. The products are made in the USA with hazard free materials, and designed to offer the exceptional durability required for home use, reliability, and a low cost of ownership, and to yield data streams that contain more than just pulse rate and SpO2, but also include added-value data to help eHealth providers to manage their devices and provide support to patients in their homes. Nonin has also implemented special programs to simplify logistics and improve their customers’ workflow.
 Headquartered in Minneapolis, Minnesota, with an additional distribution and service center in Amsterdam, the Netherlands, Nonin sells its products to health professionals and consumers in more than 125 countries and has more than 100 OEM partners worldwide. Founded in 1986 by Engineer Phil Isaacson, who serves today as the company’s managing director and chief technology officer, and three other engineers, Nonin Medical’s name was chosen to emphasize the company’s commitment to developing noninvasive medical monitoring solutions. Nonin Medical, Inc. invented the finger pulse oximeter, and is a global leader in designing and manufacturing noninvasive medical monitoring solutions, with product offerings including pulse oximeters, regional oximeters, capnographs, sensors, software and accessories.
Headquartered in Minneapolis, Minnesota, with an additional distribution and service center in Amsterdam, the Netherlands, Nonin sells its products to health professionals and consumers in more than 125 countries and has more than 100 OEM partners worldwide. Founded in 1986 by Engineer Phil Isaacson, who serves today as the company’s managing director and chief technology officer, and three other engineers, Nonin Medical’s name was chosen to emphasize the company’s commitment to developing noninvasive medical monitoring solutions. Nonin Medical, Inc. invented the finger pulse oximeter, and is a global leader in designing and manufacturing noninvasive medical monitoring solutions, with product offerings including pulse oximeters, regional oximeters, capnographs, sensors, software and accessories.
For more information about Nonin OEM products, visit:
http://nonin.com/oem
For more information about Nonin’s Model 3231 and 3230 finger pulse oximeters for eHealth/OEM applications, visit:
http://nonin.com/ehealth
Sources:
Nonin Medical, Inc.
Image Credits:
Nonin Medical, Inc.