 ADMA Biologics, Inc. will present a poster entitled “Treatment of Normal and Immune Suppressed Cotton Rats with IVIG Containing High Titer Neutralizing Anti-RSV Antibody” that describes the efficacy of RI-002, a polyclonal IVIG product, in animal modes of the human Respiratory Syncytial Virus infection. The poster will be presented at the 9th International Respiratory Syncytial Virus (RSV) Symposium in Cape Town, South Africa.
ADMA Biologics, Inc. will present a poster entitled “Treatment of Normal and Immune Suppressed Cotton Rats with IVIG Containing High Titer Neutralizing Anti-RSV Antibody” that describes the efficacy of RI-002, a polyclonal IVIG product, in animal modes of the human Respiratory Syncytial Virus infection. The poster will be presented at the 9th International Respiratory Syncytial Virus (RSV) Symposium in Cape Town, South Africa.
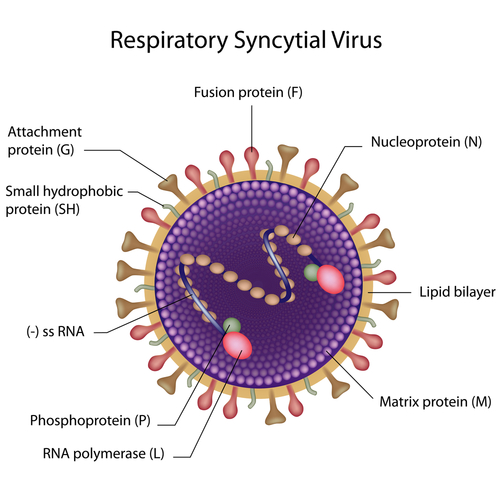
RI-002 is an Intravenous Immune Globulin (IGIV) isolated from human plasma with antibodies against respiratory syncytial virus (RSV), as well as antibodies against other infectious agents, including Streptococcus pneumoniae, H. influenza type B, Cytomegalovirus (CMV), measles and tetanus.
In the poster, the company will present their results showing RI-002 administration reduced viral load. Furthermore, prevented RSV infections in the lungs of immune suppressed cotton rats by also impairing viral dissemination to peripheral organs. Additionally, the poster includes technical information regarding antibody titer composition and how antibody levels change with RI-002 administration in IVIG and other respiratory viruses.
[adrotate group=”3″]
Jimmy Mond, M.D., Ph.D., Chief Medical and Scientific Officer for ADMA Biologics commented, “The data on RI-002 demonstrated a 99.9% reduction in viral load in both the lung and nasal tissue of cotton rats, an animal model regarded as a surrogate model for human responses to RSV infection. RI-002 not only eliminated viral load in the lung and nasal tissue but also significantly reduced RSV dissemination to the peripheral organs in the immune suppressed animals, as measured by PCR analysis. Further laboratory analysis of the composition of RI-002 demonstrated that the product contains not only high antibody titers to RSV but also contains significantly elevated levels of antibodies to other respiratory viruses.”
Specifically, the company’s findings report 99.9% reduction of viral load in lungs and nasal tissue of both normal and immune suppressed cotton rats upon RI-002 administration. This new data is particularly important, as drug development companies such as ADMA are seeking to develop new, next-generation therapies that can improve outcomes for patients with Respiratory Syncytial Virus infection.