 In a new study entitled “Secreted CLCA1 modulates TMEM16A to activate Ca2+-dependent chloride currents in human cells,” a research team uncovered an interaction between two factors, CLCA1 and TMEM16A, which may underlie the overproduction of mucus in asthma and chronic obstructive pulmonary disease. The study was published in the journal eLife.
In a new study entitled “Secreted CLCA1 modulates TMEM16A to activate Ca2+-dependent chloride currents in human cells,” a research team uncovered an interaction between two factors, CLCA1 and TMEM16A, which may underlie the overproduction of mucus in asthma and chronic obstructive pulmonary disease. The study was published in the journal eLife.
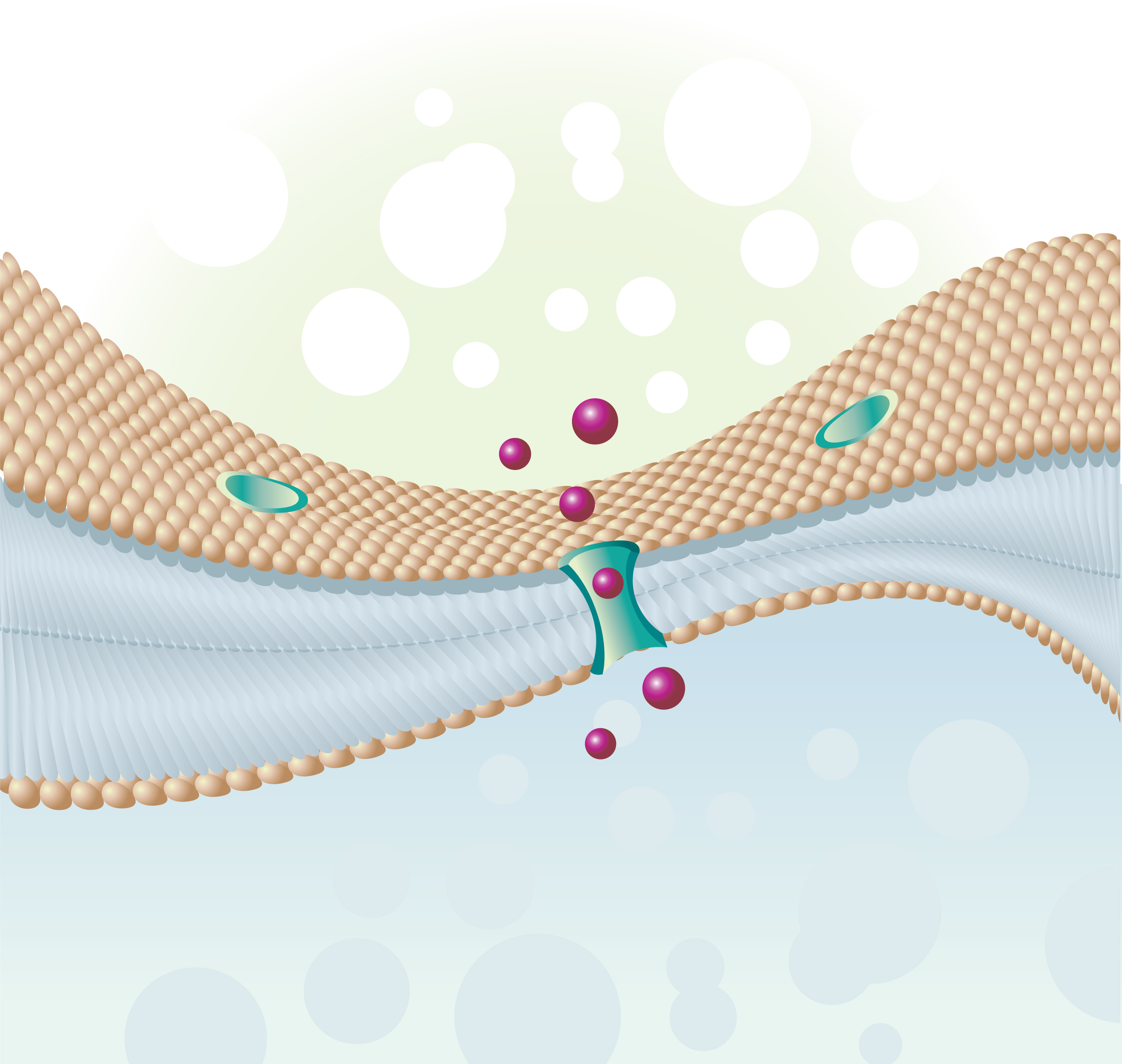
Both asthma and chronic obstructive pulmonary disease (COPD) are characterized by excess mucus production. Previous studies identified members of the calcium-activated chloride channel regulator family as highly expressed in the mucosal epithelia, leading to increased mucus production in diseases such as asthma and COPD. Notably, researchers identified CLCA1 as a channel placed in cell membrane, specifically an ion channel, i.e., regulating the entry and exit of charged molecules, such as chloride (Cl–). However, further investigation revealed that CLCA proteins are secreted, soluble proteins and were wrongly identified as calcium-activated chloride channels.
The actual channel, TMEM16A, was later identified in mammalian cells lining the epithelia of airways and smooth muscle, and was identified as responsible for mucus-overproduction. The link between CLCA1 and TMEM16A, however, remained unknown.
In this study, a research team at the Washington University School of Medicine in St. Louis tackled this question, discovering that CLCA1 expression increases the levels of TMEM16A channels at the cell surface. The authors detailed the mechanism and discovered CLCA1 works as a stabilizer of TMEM16A on surface of cells, locking the receptor and leading to enhanced ion current.
[adrotate group=”11″]
The team highlighted that their study is the first establishing the link between CLCA1 and TMEM16, and they suggest that the cooperative partnership between these two factors potentially determines the pathophysiology of many diseases, including asthma, COPD, cystic fibrosis and even certain cancers.
Thomas J. Brett, PhD, assistant professor at the Department of Cell Biology and Physiology, Washington University School of Medicine commented in a press release, “The new study lays the groundwork for developing treatments for diseases such as asthma, COPD, cystic fibrosis and even certain cancers,” said senior author. “It also solves a 20-year mystery about the role of a protein that has long been associated with these diseases.”