Researchers at the University of Alabama at Birmingham Division of Pulmonary, Allergy and Critical Medicine are investigating whether a one-way valve implanted in severe emphysema patients’ lungs of will help improve lung function. These valves are one of several non-surgical techniques under development for reducing reduce lung volume.
Some 4.9 million Americans have been diagnosed with emphysema, a form of Chronic Obstructive Pulmonary Disease (COPD) that causes airway constriction and blockage resulting in shortness of breath, chronic productive coughing that produces large amounts of mucus, chest tightness and/or wheezing and other symptoms. Labored breathing can limit an individual’s activity, making even basic activities like walking, cooking, or taking care of themselves difficult or impossible, diminishing their quality of life and putting extra strain on the heart.
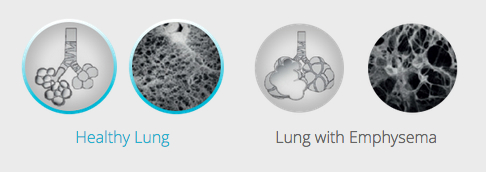
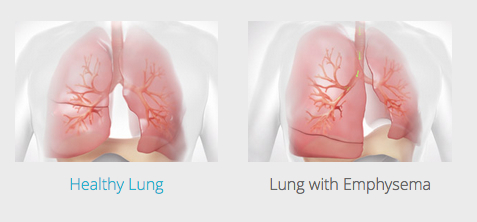
The leading cause of emphysema is cigarette smoking, which damages air sacs in the lung called alveoli. The sacs fill with air that the body is unable to exhale, which causes the lungs to expand. That in turn flattens the diaphragm — the primary muscle used for breathing. The flattened diaphragm is unable to function properly, making it extremely difficult for the individual to breathe.
One longstanding emphysema therapy is lung-volume-reduction surgery, in which over-inflated, diseased parts of the lung are surgically cut away, allowing the lung to return to a more normal size and the diaphragm to return to normal function. Surgery is effective, but not commonly done in the United States due to perceived risks for pulmonary or cardiac complications.
 “The idea behind all lung-volume-reduction procedures is to reduce the volume in the lung and allow the diaphragm to return to its normal shape and function,” says Mark Dransfield, M.D., associate professor in the Division of Pulmonary, Allergy and Critical Care Medicine and medical director of the UAB Lung Health Center. “We’re looking for a less invasive way to achieve that goal without the risks inherent in surgery.”
“The idea behind all lung-volume-reduction procedures is to reduce the volume in the lung and allow the diaphragm to return to its normal shape and function,” says Mark Dransfield, M.D., associate professor in the Division of Pulmonary, Allergy and Critical Care Medicine and medical director of the UAB Lung Health Center. “We’re looking for a less invasive way to achieve that goal without the risks inherent in surgery.”
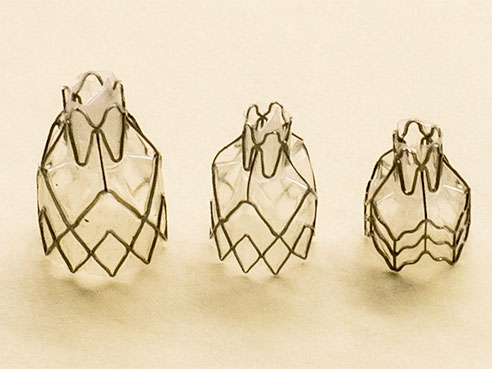
The current UAB trial is called the LIBERATE Study, using the experimental Zephyr endobronchial valve — a one-way valve that blocks airflow to the diseased region of the lung to allow healthy regions to expand and function more efficiently, and currently limited by US law to investigational use. The device’s developer, Redwood City, California and Neuchâtel, Switzerland-based PulmoniX, says patients with severe emphysema fitted with the Zephyr valve will experience a significant improvement in lung function and quality of life, with substantially improved FEV1 and sustained functional outcomes, clinically significant improvement in exercise tolerance, and long-term survival benefit through five years.
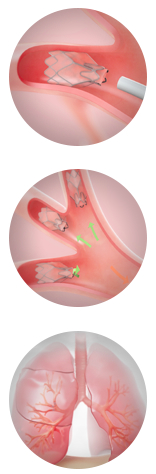 The experimental one-valves are being used in a study to seal off diseased part of the lungs in emphysema patients. “The idea behind all lung-volume-reduction procedures is to reduce the volume in the lung and allow the diaphragm to return to its normal shape and function,” says Dr. Dransfield in a UAB release. “We’re looking for a less invasive way to achieve that goal without the risks inherent in surgery.”
The experimental one-valves are being used in a study to seal off diseased part of the lungs in emphysema patients. “The idea behind all lung-volume-reduction procedures is to reduce the volume in the lung and allow the diaphragm to return to its normal shape and function,” says Dr. Dransfield in a UAB release. “We’re looking for a less invasive way to achieve that goal without the risks inherent in surgery.”
Using a standard bronchoscope, Zephyr valves are delivered to target airways using a flexible delivery catheter. Once implanted, the one-way valve prevents airflow into the diseased region, while allowing trapped air and fluids to escape. Dr. Dransfield has implanted the valve in four patients with severe emphysema thus far. Typically, a patient will have four or five valves in one side of a lung. Not all severe emphysema patients will be suitable candidates for the study. Eligibility requirements include but are not limited to: being diagnosed with emphysema; non-smoking for > 4 months before screening examination, and agreement to participate in a pulmonary rehabilitation program, both before and after study enrollment.
A brief screening survey to learn whether you may be an eligible candidate for the strial can be found here:
https://pulmonx.com/us/liberate-endobronchial-valve-study/
As with any clinical study, the LIBERATE Study involves potential risks. Prior to participation, your doctor will review benefits and risks that may be associated with device and study. For more information, contact the study site closest to you or call 1-888-248-LUNG (1-888-248-5864). Additional information on the LIBERATE Clinical Study is available at http://www.clinicaltrials.gov.
“Patients need to have enough healthy lung tissue so that the blockage of the most diseased and damaged areas and the reduced lung volume will allow the healthier areas to function more normally,” Dr. Dransfield notes.
Results of BeLieVeR-HIFi trial, the first double-blinded, randomized, controlled clinical trial to assess the impact of the Zephyr Endobronchial Valve (EBV) for the treatment of emphysema, were presented last September 9 at the 2014 European Respiratory Society Conference in Munich by Nicholas S. Hopkinson, M.D. , of The Royal Brompton & Harefield NHS Foundation Trust in London.
 Dr. Hopkinson is clinical lead for chronic obstructive pulmonary disease (COPD) at Royal Brompton Hospital, where his responsibilities include systematic evaluation of patients within a multidisciplinary team and addressing issues including hypoxia, recurrent exacerbations, alpha one antitrypsin deficiency and early onset disease. The service ensures that COPD patients have access to novel techniques for lung volume reduction both surgical and bronchoscopic. Dr. Hopkinson has established a smoking cessation clinic within the Trust and is responsible for developing pulmonary rehabilitation services as well as the Trust’s Breathe Easy Support Group. He is a member of the London Respiratory Clinical Leadership Group and active in tobacco control advocacy, including being a member of the advisory group of The Smoke Free Action Coalition.
Dr. Hopkinson is clinical lead for chronic obstructive pulmonary disease (COPD) at Royal Brompton Hospital, where his responsibilities include systematic evaluation of patients within a multidisciplinary team and addressing issues including hypoxia, recurrent exacerbations, alpha one antitrypsin deficiency and early onset disease. The service ensures that COPD patients have access to novel techniques for lung volume reduction both surgical and bronchoscopic. Dr. Hopkinson has established a smoking cessation clinic within the Trust and is responsible for developing pulmonary rehabilitation services as well as the Trust’s Breathe Easy Support Group. He is a member of the London Respiratory Clinical Leadership Group and active in tobacco control advocacy, including being a member of the advisory group of The Smoke Free Action Coalition.
[adrotate group=”3″]
In the BeLieVeR-HIFi study, the third randomized clinical trial of the Zephyr device, patients with emphysema receiving the valves demonstrated statistically and clinically significant improvements in lung function, gas trapping and gas exchange, as well as in exercise capacity measured on a cycle ergometer. The mean improvement in FEV1, a measure of pulmonary function, was 25% in treated patients. In addition, the procedure showed an acceptable safety profile, with clinically significant improvements in walking distance and quality of life.
“Many patients with emphysema remain very disabled because even optimal medical therapy is of limited benefit. However, this study shows clearly that in appropriately selected individuals with emphysema, endobronchial valves offer a real prospect for improving lung function, exercise capacity, and quality of life,” Dr. Hopkinson, who served as a principal investigator for the trial, commented in a PulmonX release.
Previous studies of the device had demonstrated sustained improvement and a survival benefit was later observed in patients without cross-communication between the different regions of their lungs. The BeLieVeR-HIFi study deliberately targeted only this specific type of patient to prospectively confirm this finding.
“The results of this study add to the growing body of evidence for the Zephyr valve, which has now been used to treat approximately 8,500 patients with emphysema around the world,” said Oern Stuge, M.D., Interim CEO, PulmonX. “We look forward to continued validation of the effectiveness of the therapy in appropriately selected and treated patients with the conclusion of a number of ongoing studies.”
While the Zephyr procedure is not a cure for emphysema; Dr. Dransfield observes that the clinical studies in Europe have indicated the majority of patients do experience significant improvement in lung function, exercise tolerance and quality of life, with more than 25,000 Zephyr valves having been implanted outside the United States over the past 10 years.
Results from the LIBERATE Study will be used as part of the submission seeking U.S. approval for the Zephyr Endobronchial Valve from the FDA.
For more information about the LIBERATE clinical trial, call the UAB Lung Health Center at 205-934-5555
visit http://www.pulmonx.com
or call 888-248-5864
Sources:
University of Alabama at Birmingham
PulmoniX
The Royal Brompton & Harefield NHS Foundation Trust
Image Credits:
University of Alabama at Birmingham
PulmoniX
The Royal Brompton & Harefield NHS Foundation Trust


What if any studies have been done for the Zephyr valve in relation to sea level breathing and higher altitude breathing (4600 ft or more) How can you tell if you valves have become ineffective?
Would this treatment be any good for people with lpf