In a new study entitled “Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening,” researchers identified mutations in two genes, PARN and RTEL1, which are associated with familial pulmonary fibrosis. Additionally, the team found that mutation carriers have shortened telomeres, providing a new link between lung fibrosis and telomere dysfunction. The study was published in the journal Nature Genetics.
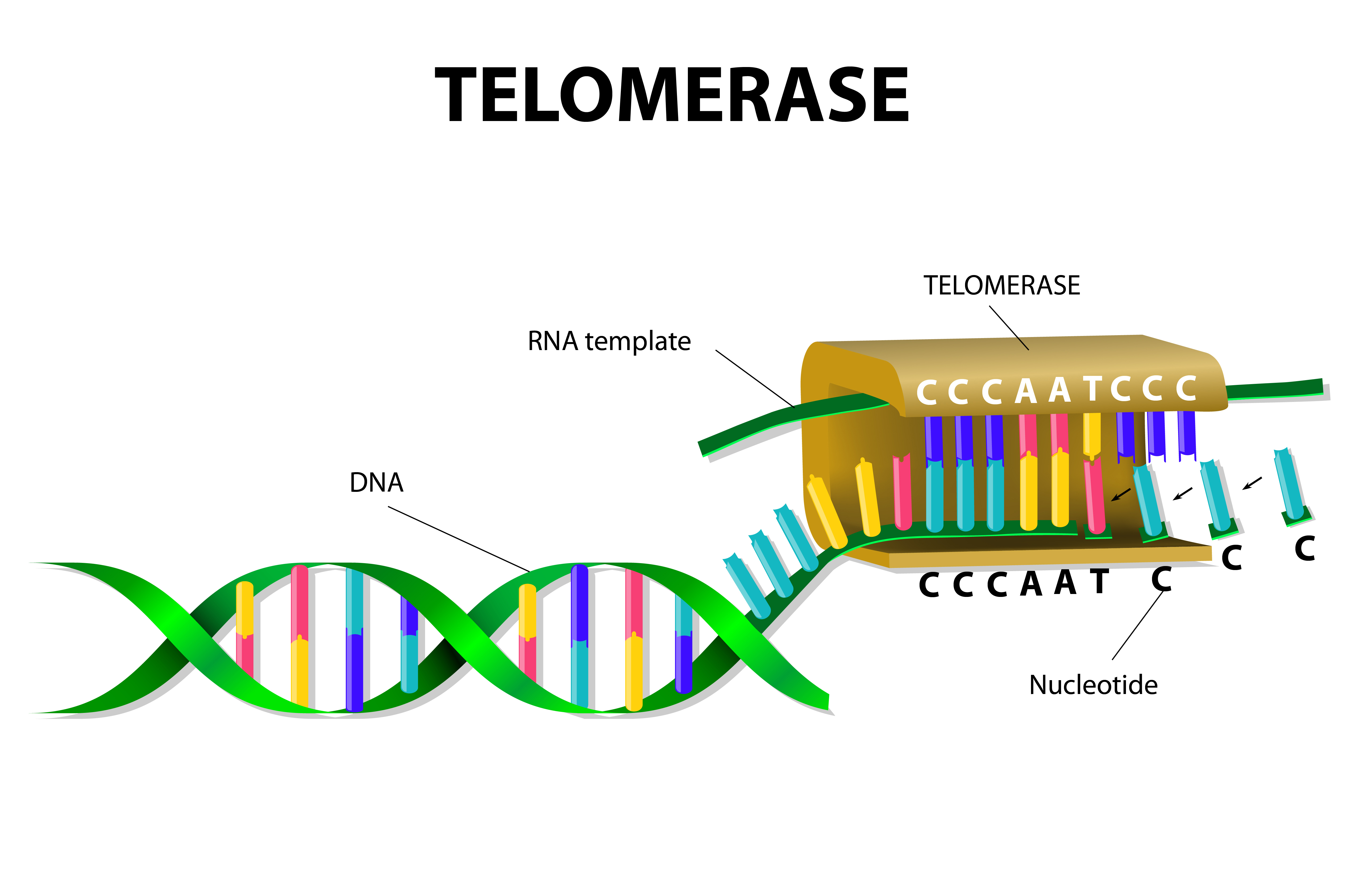
Idiopathic pulmonary fibrosis (IPF) is a chronic disease characterized by scarring of the lungs, leading to a progressive decline in lung function and is ultimately fatal (life expectancy is usually 2 to 3 years after diagnosis). While some mutations were identified as playing a role in disease risk, these genetic variants are only observed in a small fraction of IPF cases. Notably, however, these mutations affect genes that are linked to the human telomerase, including the human telomerase RNA component (TERC) and dyskerin (DKC1) but also surfactant proteins (SFTPC and SFTPA2), essential for lung function.
Since familial cases of pulmonary fibrosis exhibit short telomere lengths that are unrelated to these previous identified mutations affecting telomerase, in this study researchers aimed to identify other genes affecting telomere maintenance. To this end, a research team at UT Southwestern Medical Center performed whole-exome sequencing (i.e., sequencing all the protein-coding genes in a genome) in DNA samples from 99 families with familial pulmonary fibrosis, who lacked any of the already known mutations.
The team identified mutations in two new genes – PARN and RTEL1 – occurring in 12% of the families analyzed. They measured telomere length and observed that mutations in both genes are associated with shortened telomeres. These results strengthen the link between telomere dysfunction and the risk for lung fibrosis and the identification of new genes underlying increased disease risk is essential for pursuing new therapeutics.
[adrotate group=”7″]
Dr. Christine Kim Garcia, Associate Professor of Internal Medicine and with the Eugene McDermott Center for Human Growth and Development and study lead author noted, “Although RTEL1 had been previously linked to telomere biology, our finding that PARN was involved in telomere regulation and human disease was completely unexpected. Our ultimate goal is to gain a full understanding of what causes the genetic form of this disease so that effective medications can be developed.”