Baltimore based advanced cancer genome analysis and testing services firm Personal Genome Diagnostics, Inc. (PGDx) last month launched its LungSelect product that incorporates a proprietary technology for identifying the most common, clinically actionable genetic alterations in the plasma of non-small cell lung cancer (NSCLC) patients. Dr. Theresa Zhang, PhD, PGDx’s Vice President of Research Services, described and explained LungSelect in a presentation on June 12 entitled: “Identify Clinically Actionable Sequence Mutations and Translocations in the Plasma of Lung Cancer Patients without Invasive Biopsies,” at the GTCBio Companion Diagnostics Conference, held June 11-12, 2015 at the Hyatt Regency Mission Bay in San Diego.
Personal Genome Diagnostics was founded by leading researchers from Baltimore’s Johns Hopkins University, and provides advanced cancer genome analysis to help researchers identify elusive cancer-related genetic changes. The company’s services further the understanding of cancer and facilitate development of new diagnostics and therapeutics through its pioneering research approaches and novel technologies. PGDx uses advanced genomic methods and its deep expertise in cancer biology to identify and characterize the unique genomic alterations in tumors.
In response to the increasing availability of targeted NSCLC therapies, PGDx notes that clinical guidelines recommend molecular testing of relevant genes to identify candidates for targeted therapy. However, an estimated one-third of NSCLC patients lack adequate tissue samples for molecular testing and therefore cannot be considered for the new targeted treatments.
To address that shortcoming, the plasma-based LungSelect test enables testing of all NSCLC patients for relevant sequence mutations, insertions and deletions, and genomic rearrangements, including patients who may have acquired new mutations post-treatment and those with multiple tumor sites.
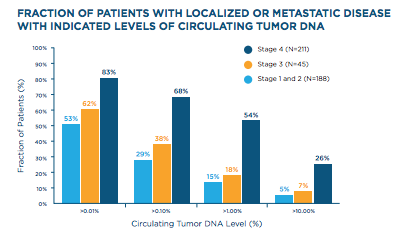
PGDx’s LungSelect technology simultaneously identifies somatic sequence mutations and translocations that can be treated with agents currently approved by the FDA or ones that are in clinical trials, including most defined in the NCCN Guidelines. The company says LungSelect is able to detect sequence mutations and tr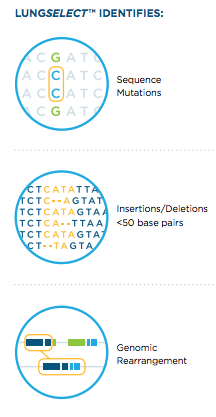 anslocations down to 0.02% and 0.1% circulating tumor DNA levels respectively, with sensitivity greater than 90 percent and positive predictive value greater than 99 percent, noting that the majority of NSCLC patients with advanced disease have mutations detectable at these circulating tumor DNA levels (Bettegowda et. al., Sci Transl Med. 2014 Feb 19;6(224):224ra24).
anslocations down to 0.02% and 0.1% circulating tumor DNA levels respectively, with sensitivity greater than 90 percent and positive predictive value greater than 99 percent, noting that the majority of NSCLC patients with advanced disease have mutations detectable at these circulating tumor DNA levels (Bettegowda et. al., Sci Transl Med. 2014 Feb 19;6(224):224ra24).
“The rapid advances in targeted cancer therapies for treating NSCLC make the availability of LungSelect especially timely,” says Dr. Zhang. “All NSCLC patients will now have the opportunity to be tested to see if new life-extending therapies may be relevant to their cancer, regardless of whether or not tumor tissue is available. PGDx is a pioneer in liquid biopsies for cancer, and we are pleased to offer this informative new test for NSCLC, which is the single largest cause of cancer deaths in the U.S.”
The LungSelect test is analyzed in PGDx’s CLIA laboratory, with results typically available within approximately two weeks. For more information, visit http://www.mypersonalgenome.com/.
In summary, benefits of using plasma for cancer mutation analyses include:
• A non-invasive, liquid biopsy assay will be helpful in non-small cell lung cancer patients where:
• Biopsy not available, insufficient or exhausted before molecular testing can be performed
• Tissue-based molecular testing failed due to poor quality DNA from tissue biopsy
• Suspicion that the tumor may have acquired mutations as result of treatments since the original biopsy was taken
• Desire to detect genetic alterations in multiple lesions simultaneously
• Desire to avoid the risks and costs associated with re-biopsy
For more information, visit:
http://www.personalgenome.com
Sources:
Personal Genome Diagnostics Inc.
GTCBio Companion Diagnostics Conference
Image Credits:
Personal Genome Diagnostics Inc.
GTCBio Companion Diagnostics Conference