New Zealand-based Adherium (NZ) Limited (formerly known as Nexus6) has entered a long term partnership with London, U.K. based multinational biopharmaceutical company AstraZeneca in what the company believes is a world-first commercial arrangement combining digital health technology with blockbuster inhaler medications to improve health outcomes for patients with respiratory conditions. AstraZeneca, formed in 1999 through the merger of Astra AB of Sweden and Zeneca Group PLC of the UK, now operates in more than 100 countries and is focused on discovery, development and commercialization of prescription medicines, primarily for treatment of cardiovascular, metabolic, respiratory, inflammation, autoimmune, oncology, infection and neuroscience diseases. AstraZeneca’s medicines are used by millions of patients worldwide.
Under a long term Supply Agreement, Adherium will supply innovative new devices and sensors that AstraZeneca will incorporate within its global patient support programs for patients with the respiratory disorders Chronic Obstructive Pulmonary Disease (COPD) and asthma.
AstraZeneca affirms strategic partnering and business development is a key accelerator of its corporate strategy. The company has been active in building early stage biotech and academic alliances in recent years and says it wants to pursue more of these, alongside in-licensing and acquisitions to strengthen its pipeline and broaden the range of treatment options it offers for physicians and patients.
“We are very pleased to be working with AstraZeneca,” says Adherium Managing Director and CEO Garth Sutherland. These agreements represent a major advance in the treatment and management of respiratory disease, and will make a fundamental difference to the quality of life for people with asthma and COPD.”
Adherium has developed and refined its device monitoring technology over the past 15 years, and has collaborated with a broad range of academic partners to demonstrate its devices’ robustness and effectiveness. The company notes that overall, fewer than 50 percent of asthma patients adhere to their prescribed preventative medications. However, they say addition of an Adherium Smartinhaler has been clinically proven to increase adherence by up to 59 percent in adults and 180 percent in children with asthma (see clinical studies referenced below). They also cite clinical evidence that in adults, using a Smartinhaler can reduce severe episodes by 60 percent which represents a profound improvement over current best practice.
In a study published in the journal Lancet Respiratory Medicine, entitled: “The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomized controlled trial“ (Lancet Respir Med. January 21 2015. http://dx.doi.org/10.1016/S2213-2600(15)00008-9), coauthors Amy H. Y. Chan, BPharm; Alistair W. Stewart, BSc; Jeff Harrison, PhD; Prof. Carlos A. Camargo Jr, MD; Prof. Peter N Black, FRACP; and Prof. Edwin A Mitchell, FRSNZ; note that “sub-optimum adherence to preventive asthma treatment is associated with substantial morbidity and mortality, yet adherence often remains poor.” The researchers’ objective was to investigate whether use of an inhaler with audiovisual reminders would lead to improved adherence and asthma outcomes in school-aged children presenting at emergency departments with asthma exacerbations.
The investigators conducted a randomized controlled trial (registered with the Australian New Zealand Clinical Trials Registry, number ACTRN12613001353785) in patients aged 6-15 years who had attended the regional emergency department in Auckland, New Zealand presenting with asthma exacerbations and who were on regular inhaled corticosteroids. Randomly assigned patients received an electronic monitoring device (Smartinhaler) for use with their preventer inhaler with the audiovisual reminder functions either enabled to support adherence to inhaled corticosteroids (intervention group) or disabled (control group). Participants were followed up every 2 months for 6 months.
Primary outcomes of the study were adherence to preventive inhaled corticosteroids and number of days absent from school for any reason, with asthma control assessed as a secondary outcome. All analyses were done in the intention-to-treat population. The researchers randomly assigned 220 patients, 110 to the intervention group and 110 to the control group, and found that median percentage adherence was 84 percent in the intervention group, compared with 30 percent in the control group. The proportion of days absent from school for any reason was 19 percent in the intervention group and 17 percent in the control group.
Based on the study findings, the investigators conclude that use of an electronic monitoring device with an audiovisual reminder led to significant improvements in adherence to inhaled corticosteroids in school-aged children with asthma, and could be beneficial for improving asthma control in patients with whom poor asthma control is related to poor adherence. The study was funded by the Health Research Council of New Zealand and Cure Kids.
The partnership between Adherium and AstraZenica establishes Adherium as a leading player in digital health technologies that can transform the management of chronic respiratory diseases, and demonstrates AstraZeneca’s commitment to its Intelligent Pharmaceuticals program by being the first company to execute a commercial agreement for digital technology complementary to its inhalation products.
The collaboration also validates the commercial importance of Adherium’s technology in the global digital health market.
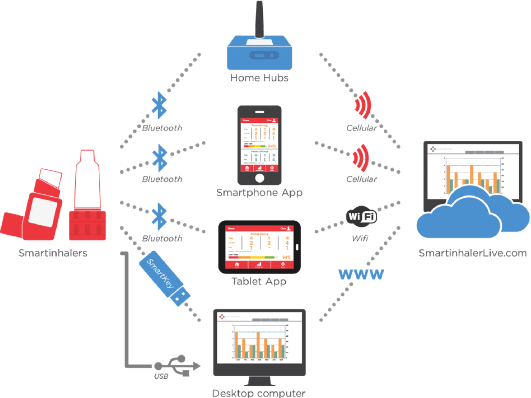
The company has developed a wide range of electronic monitors (the Smartinhaler product range) that record time and date of the use of an individual inhaler and transmits that information to the patient’s mobile device and to a treating physician. This provides objective data on inhaler medication use to patients, physicians, and caregivers.
AstraZeneca reports that it has already successfully used this technology in clinical evaluations and clinical trials, and piloted its use in programs to support patients in managing their conditions. AstraZeneca plans to use Adherium device technology as the pivotal component of its innovative global patient support programs.
Initially, they say the technology will be used to monitor patients’ actual use of therapy and to provide personalized advice to patients based on their individual conditions and medication use. In future, developments are projected to include additional sensors designed to monitor a patient’s condition and potentially to assess a patient’s personalized risk factors.
Adherium Chairman Dr. Doug Wilson observes: “This partnership signifies a change in the way we approach the treatment of respiratory disease. By providing objective and accurate data on a patient’s medication usage, we can now for the first time develop treatment plans tailored to each patient, and in doing so dramatically improve their quality of life.”
The company’s proprietary Smartinhaler platform includes of a range of approved medical devices that attach to prescription inhalers to record medication usage patterns. The devices track date and time of patient use and transmit audio and visual reminders to the patient when they miss a critical dose of preventative medication. The devices also provide warnings to the patient when their rescue medication use indicates their condition is becoming uncontrolled. The Smartinhaler devices automatically transmit this data using wireless communications technologies to a smartphone app, or a remote monitoring hub, or personal computer from where the data can be shared with the patients’ clinician for additional support.
Adherium notes that currently clinicians have no system to record medication usage and consequently are obliged to rely heavily on subjective patient feedback, which can be inaccurate. Having access to accurate and objective data such as Smartinhaler provides will enable physicians to identify sub-optimal medication adherence and to suggest more effective treatment plans for patients, resulting in a reduction of significant financial costs associated with suboptimal medication adherence has on healthcare systems worldwide.
Clinical outcomes data have proved that the Smartinhaler platform can improve adherence by up to 59 percent in adults and 180 percent in children with asthma, and additionally that severe episodes were reduced by 60 percent in adults with asthma — both groups benefiting from improved quality-of-life as a result of improved adherence, demonstrating a substantial gain over current best practice treatment.
An earlier study published last December in the Journal of Allergy and Clinical Immunology (JACI), entitled “Inhaler reminders improve adherence with controller treatment in primary care patients with asthma“ (Journal of Allergy and Clinical Immunology, Volume 134, Issue 6, December 2014, Pages 1269-1270 doi:10.1016/j.jaci.2014.05.041), discovered a ground-breaking increase in adherence with asthma medications for patients provided with a Smartinhaler, manufactured Adherium, which was at the time known as Nexus6 Ltd.
The research was conducted by leading respiratory investigators Dr. Juliet M. Foster and Professor Helen K. Reddel of the Woolcock Institute of Medical Research’s Clinical Management Group at the University of Sydney in Sydney, Australia, with coauthors Tim Usherwood, BSc, MD, BSb; Lorraine Smith, PhDc; Susan M. Sawyer; MBBS, MDd; Wei Xuan, MSc, MAppStat, PhDg; of various Australian institutions and Cynthia S. Rand, PhD of the Department of Pulmonary and Critical Care Medicine at Johns Hopkins School of Medicine in Baltimore, Maryland. Their study also showed that patients who were provided with an interventional Smartinhaler were much less likely to experience a severe asthma exacerbation compared with patients managed under usual care or usual care with personalized adherence discussions.
At six months, the investigators found that Smartinhaler had doubled adherence to prescribed maintenance treatment, an outcome attributed to proprietary features on the Smartinhaler, including audio and visual reminder technology, and also wireless communications to a Cloud-based analytics and reporting website.
Furthermore, Drs. Foster and Reddel measured these outcomes in a real world setting in which patients were provided the Smartinhaler by their General Practitioner with little intervention from the investigation team. The coauthors conclude that Inhaler reminders offer an effective strategy for improving adherence in primary care compared with a behavioral intervention or usual care, although this may not be reflected in differences in day-to-day asthma control, noting that “Inhaler reminders offer an effective strategy for improving adherence in primary care compared with a behavioral intervention or usual care, although this may not be reflected in differences in day-to-day asthma control.”
Adherium/Nexus6 Chairman Dr. Doug Wilson commented at the time: “These findings represent one of the biggest breakthroughs in respiratory care since the launch of combination therapies in the ’90s. Outcomes are crucial differentiators in the respiratory world where up to 20 new inhaled products recently obtained or are close to market approval in the US, EU or Japan. Foster and Reddels’ independent findings prove that Nexus6’s Smartinhaler fundamentally shifts patient behavior regardless of standards of care, with a major potential impact for disease management.”
For more information, visit:
http://www.adherium.com
Sources:
Adherium Limited
AstraZenica
Lancet Respiratory Medicine
Journal of Allergy and Clinical Immunology
Image Credits:
Adherium Limited