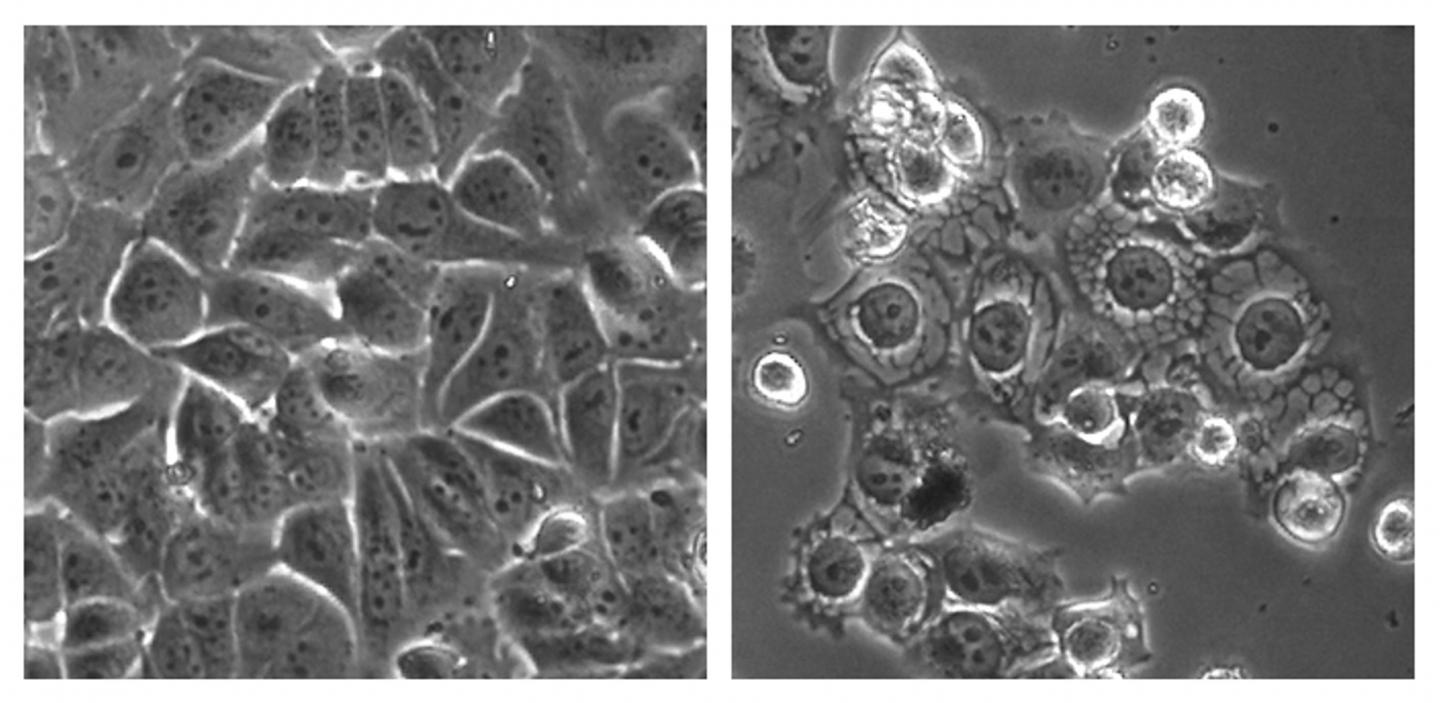
In the photo shown, cancer cells were treated with a control (left) and the overstimulating compound MCB-613 (right).
CREDIT
Lei Wang
A new, potentially promising drug overstimulates proteins that are needed in order for tumors to grow, according to a new study that appeared in the August 10 issue of Cancer Cell. Baylor College of Medicine researchers demonstrated that they could over-stress cancerous cells, effectively blocking their growth in mice with experimental cancer. The method could be effective for many different cancer types, including lung cancer.
Cancer cells have oncogene mutations. Oncogenes are genes that allow a cell to become cancerous and make it grow and survive. Steroid receptor coactivator (SRC) oncogenes are a specific type of oncogene that might be particularly useful as a drug target because they seem to allow the spread of cancer throughout the body and promote resistance to anti-cancer drugs. In a previous study, co-senior study author David Lonard and co-senior study author Bert O’Malley of Baylor College of Medicine found several different molecules that block SRC, kill different cancer cells, and stop tumor growth in animal models of cancer.
The researchers decided to test the reverse concept. Rather than blocking SRC in the current study, they sought to over-stimulate it.
They screened thousands of compounds and eventually discovered the SRC activator MCB-613. The scientists gave MCB-613 to 13 mice with experimentally-induced breast cancer. Remarkably, MCB-613 wiped out almost all of the cancer growth but did not cause side effects typical of cancer drugs. Fourteen mice that did not receive the drug continued to have tumor growth.
“No prior drug has been previously developed or proposed that actually stimulates an oncogene to promote therapy,” remarked Lonard. “Our prototype drug works in multiple types of cancers and encourages us that this could be a more general addition to the cancer drug arsenal.”
The drug seemed to work by building up proteins in a structure within the cell called the endoplasmic reticulum. This “stressed out” the cancerous cell and caused it to die. It therefore seems that by over-stimulating SRC, a cancerous cell becomes overworked to the point of death, instead of being stimulated to proliferate and spread.
Whether or not MCB-613 makes it to the clinic will depend on further studies and ultimately on human trials. It is also possible that the researchers will identify or develop additional molecules that act in a similar manner. Overall, the investigators are confident that their new approach will be effective for treating a variety of cancer types, including lung cancer. “We are optimistic that these drugs will eventually find their way into the clinic for use in patients,” O’Malley noted.