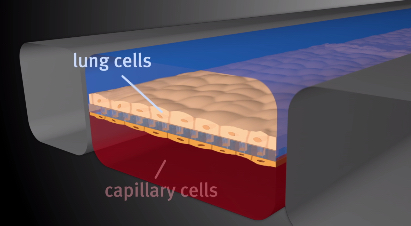
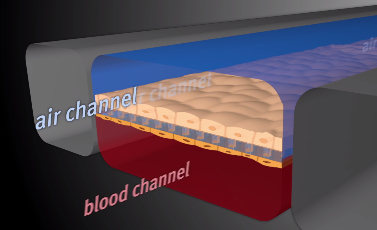
A team of researchers at Harvard University’s Wyss Institute for Biologically Inspired Engineering in Boston has leveraged its organ-on-a-chip technology in developing a microfluidic human small airway model that allows study of lung inflammatory diseases such as chronic obstructive pulmonary disease (COPD) and asthma outside the human body.
Chronic lung diseases are among the most common medical conditions worldwide, with estimates of up 30 million people in the U.S. and approximately 65 million worldwide suffering from COPD alone according to the World Health Organization. The progressive lung disease is, according to the Centers for Disease Control and Prevention (CDC), the third leading cause of mortality worldwide — including in America (138,080) — after heart disease (597,689) and cancers (574,743). The American Lung Association notes that COPD is also a major cause of disability, and while some 12 million Americans have a formal COPD diagnosis. An estimated 12-18 million more may have the disease and go undiagnosed.
COPD typically involves a combination of emphysema and chronic bronchitis, both treatment-resistant conditions, and is most commonly associated with smoking. According to the CDC, some 434,000 people die annually in the U.S. from smoking related illness.
A paper published online before print on by the journal Nature Methods on December 21 reports how the Wyss Institute developed small human airway-on-a-chip platform allows researchers to gain new insights into COPD and asthma disease mechanisms, identify novel biomarkers, and test new drug candidates outside the human body.
The Wyss researchers note in a release that COPD and asthma, both inflammatory reactions in the lung, can be dramatically exacerbated by viral and bacterial infections as well as by smoking, and that it is known that many associated disease processes occur in the conducting airway sections of the lung that shuttle air to and from the alveoli or air sacs. However, they point out that much less is known about how inflammation induces distinct pathological processes such as recruitment of circulating white blood cells and mucus buildup, which compromise the lungs of COPD and asthma patients, or how clinical exacerbations are triggered.
The Nature Methods paper, entitled “Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro” (Nature Methods 2015 doi:10.1038/nmeth.3697) describes development of a human lung ‘small airway-on-a-chip’ containing a differentiated, mucociliary bronchiolar epithelium and an underlying microvascular endothelium that experiences fluid flow, allowing analysis of organ-level lung pathophysiology in vitro rather than inhuman subjects.
(Image Credits: Wyss Institute at Harvard University)
They note that exposure of the epithelium to interleukin-13 (IL-13) reconstituted goblet cell hyperplasia, cytokine hypersecretion and decreased ciliary function of asthmatics, and that small airway chips lined with epithelial cells from COPD patients recapitulated aspects of the disease such as selective cytokine hypersecretion, increased neutrophil recruitment and clinical exacerbation by exposure to viral and bacterial infections.
“With this robust in vitro method for modeling human lung inflammatory disorders, it is possible to detect synergistic effects of lung endothelium and epithelium on cytokine secretion, identify new biomarkers of disease exacerbation and measure responses to anti-inflammatory compounds that inhibit cytokine-induced recruitment of circulating neutrophils under flow,” the coauthors observe.
 “Inspired by our past work using the organ-on-a-chip approach to model the lung alveolus, we created a new microfluidic model of the lung small airway that recapitulates critical features of asthma and COPD with unprecedented fidelity and detail. Now with this microengineered human lung small airway, we can study lung inflammatory diseases over several weeks in chips lined by cells from both normal donors and diseased patients to gain better insight into disease mechanisms, as well as screen for new therapeutics,” says Donald Ingber, M.D., Ph.D. — Founding Director of the Wyss Institute, the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
“Inspired by our past work using the organ-on-a-chip approach to model the lung alveolus, we created a new microfluidic model of the lung small airway that recapitulates critical features of asthma and COPD with unprecedented fidelity and detail. Now with this microengineered human lung small airway, we can study lung inflammatory diseases over several weeks in chips lined by cells from both normal donors and diseased patients to gain better insight into disease mechanisms, as well as screen for new therapeutics,” says Donald Ingber, M.D., Ph.D. — Founding Director of the Wyss Institute, the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Dr. Ingber is project leader of the multidisciplinary team of Wyss scientists that is at the forefront of organ-on-chip technology, and senior author of the Nature Methods paper. Demand for this type of technology is especially high since small airway inflammation can’t be adequately studied in human patients or animal models and, to date, there are no effective therapies that can stop or reverse the complex and widespread inflammation-driven processes. The “organ-on-a-chip” microchips the size of memory sticks can be used to study drug toxicity, identify potential new disease therapies, and approach clinical trials in a new way.
“To closely mimic the complex 3D cellular architecture of actual human small airways, we designed a microfluidic device that contains a fully matured human small airway epithelium with different specialized cell types exposed to air in one of its two parallel microchannels. The second channel is lined by a human vascular endothelium in which we flow medium containing white blood cells and nutrients so that the living microsystem can be maintained over weeks. We then modeled inflammatory asthma and COPD conditions by adding an asthma-inducing immune factor or by setting up the system with lung epithelial cells obtained from patients with COPD,” says Remi Villenave, Ph.D., a former postdoctoral fellow in Dr. Ingber’s group and co-first author of the paper. In both cases, the team was not only able to observe highly disease- and cell type-specific changes but could also exacerbate them with agents simulating viral or bacterial infection.
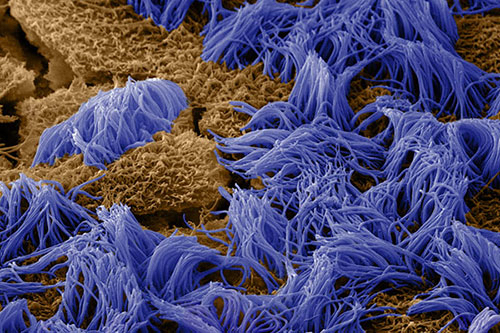
“This new organ-on-a-chip technology gives us a window on molecular-scale activities in the context of living human lung tissue. It also provides us with a handle to dissect contributions of specific cell types and biochemical factors to small airway diseases, including how circulating immune cells are recruited to inflammation sites and how compromised cilia function contributes to abnormal mucus clearance in the lungs of diseased patients,” said Kambez Hajipouran Benam, Ph.D., who also is a postdoctoral fellow working with Dr. Ingber and the study’s other co-first author.
The research team has also provided proof-of-principle that the synthetic small airway-on-a-chip can be utilized as a discovery platform for disease-specific drugs and biomarkers. Working in collaboration with two industrial partners — Pfizer and Merck Research Laboratories respectively which also helped fund the project, together with support from the Defense Advanced Research Project Agency (DARPA), the Wyss researchers showed that two drugs targeting different key molecular components of inflammatory pathways can potently suppress pathological processes in asthma and COPD-tailored small airway chips. The Wyss scientists have also identified a macrophage-recruiting factor whose levels are raised by a viral mimic in the COPD model that can be further investigated as a potential specific biomarker for viral exacerbations of COPD.
“This novel ability to build small airway chips with cells from individual patients with diseases like COPD positions us and others now to investigate the effects of genetic variability, specific immune cell populations, pharmaceutical candidates and even pandemic viruses in an entirely new and more personalized way; one that will hopefully increase the likelihood of success of future therapeutics,” Dr. Ingber observes.
Sources:
Wyss Institute for Biologically Inspired Engineering at Harvard University
Nature Methods
Centers for Disease Control and Prevention(CDC)
American Lung Association