Madison, Wisconsin-based HealthMyne announced it has received 501(k) premarketing approval from the FDA for its powerful new analytics software, clearing the way for full marketing of the software. According to the FDA, this is one of the most stringent marketing applications required by the agency, and must prove the device — or, in this case, the software — is safe and effective.
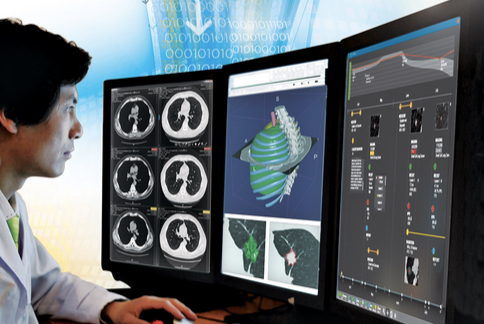
The analytics software provides radiologists and oncology clinicians with an informatics platform that combines imaging and electronic health records (EHR) information to aid in evidence-based analytics and decision-making.
The heart of the HealthMyne system is a powerful image-analysis engine that generates information such as tumor size, lung-RADS categories for use in lung cancer screening, and other advanced quantitative metrics derived from the imaging study.
Exams, test results, treatment details, and health status for each patient are viewable in a timeline-based representation which provides a longitudinal perspective with clear visual insights into a patient’s continuum of care. The timeline can include graphical representations of laboratory tests, imaging procedures, disease diagnosis and courses of therapy, along with quantitative values and plots from imaging and non-image-based analyses.
An example of a longitudinal representation would be a plot of tumor size relative to duration of a course of radiotherapy. Icons indicate dates of follow-up computer tomography (CT) scans from which tumor size can be determined. Scans can be examined by clicking on their icon and opening a viewer. Timeline elements such as analytics derived from follow-up exams can be added as they become available. With the patient’s information in one place, the need to refer back to paper notes and charts or other electronic information sources while reading and reporting on imaging exams is eliminated.
HealthMyne notes that new image characterization and tracking features are now provided in a comprehensive, quantitative imaging dashboard, which includes time-sequenced Epic EHR information. The analytics software also offers the convenience of being able to track a nodule or tumor over multiple, spatially registered imaging studies, which can reveal crucial information about whether the structure is growing, shrinking, or stable.
This window on structure change can be particularly useful in monitoring or adjusting a patient’s treatment regime, and with cancer treatment options continuing to increase in both number and specificity, the HealthMyne software can provide valuable insights that can result in improved clinical decision-making.
Cancer screening programs can also benefit from nodule characterization. For example, HealthMyne software automatically identifies nodule growth in patients participating in lung cancer screening programs — potentially an indicator of nodule malignancy, suggesting that extra scrutiny is appropriate.
“By automatically delineating the boundaries of a lung nodule [tumor], our software can make other sophisticated measurements within those boundaries — such as quantitatively describing important properties like size, shape, and texture,” HealthMyne CTO Roger Chylla said in a press release. “Physicians can examine these properties to make critical judgments about a patient’s prognosis and follow-up schedule, or how to best treat them.”
HealthMyne will be exhibiting at the annual meeting of the Society of Thoracic Radiology March 13-16 at the Hyatt Regency Scottsdale Resort & Spa at Gainey Ranch in Scottsdale, Arizona.
A brochure of the meeting’s agenda is available HERE.