Inhaled nitric oxide (iNO) treatment with the investigational medical device INOpulse system in patients with pulmonary hypertension associated with idiopathic pulmonary fibrosis (PH-IPF) has shown promising results in a Phase 2 clinical trial (NCT02267655).
Bellerophon Therapeutics will present the results on its INOpulse device at the American Thoracic Society (ATS) 113th International Conference, on May 21, in Washington, D.C., in a poster titled “Unraveling the mode of action of pulsed inhaled NO in severe IPF using Functional Respiratory Imaging (FRI).”
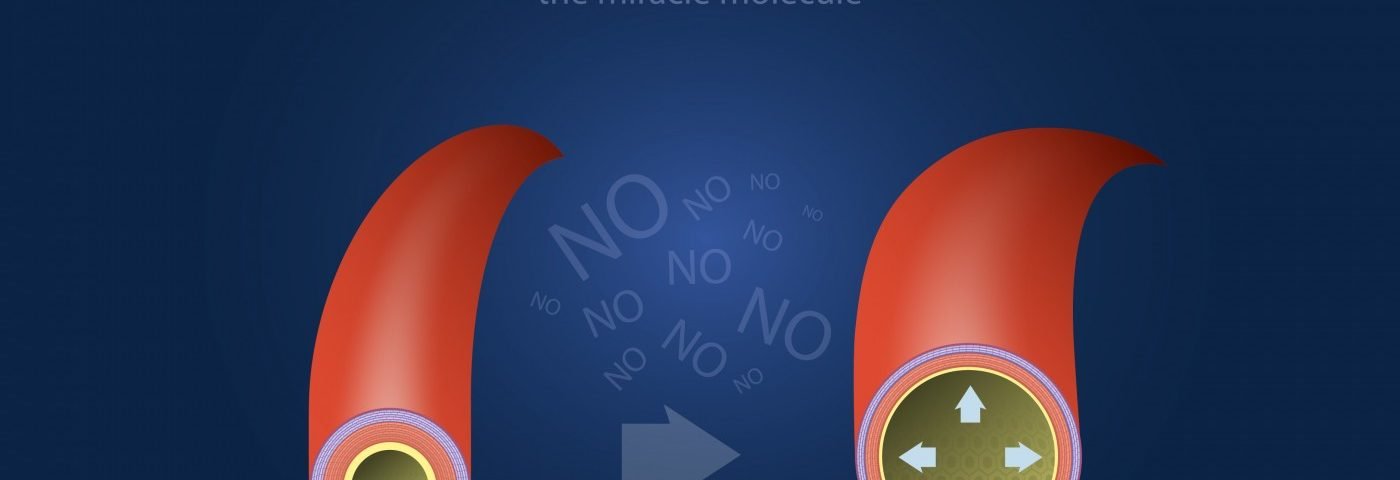
Nitric oxide is a vasodilator naturally produced by the body in the blood vessel linings, working on the smooth muscle of the blood vessels to dilate or open the arteries. Research has suggested that iNO may help open or dilate blood vessels in the lungs.
The study tested four patients with PH-IPF. In the acute phase of the study, the optimum iNO dose and the impact on hemodynamic measures was evaluated. In the chronic phase, the impact of iNO on exercise capacity was assessed using the six-minute walk test after four weeks of treatment.
All patients showed improvement in systolic pulmonary arterial pressure (sPAP; a measure of blood pressure in the arteries supplying blood to the lungs), which was reduced on average by 14%.
The patients also walked an average of 75 meters (246 feet) more at the end of four weeks of treatment in the six-minute walk test.
The results suggested that a dose of iNO safely provided a reduction in sPAP and improved patients’ physical conditions.
“The results from this study suggest that iNO allows selective vasodilation to the well-functioning parts of the lung to improve hemodynamic measures as well as exercise capacity,” said W. De Backer, MD, in a press release. De Backer, who is a director in the Department of Pulmonary Medicine, University Hospital and University of Antwerp, led the study.
“This is a potentially important advantage for pulsed iNO therapy since the currently available vasodilators that are used to treat pulmonary hypertension act systemically and have been shown to cause issues, such as worsening hypoxemia, when used on PH-IPF patients.” De Backer said.
The possible advantage of using iNO in IPF is that it appears to target the functional areas of the lungs only and does not seem to have effects in the body outside the lungs. Therefore, the treatment should not cause the side effects in IPF patients seen with some other vasodilators.
The poster presentation at the ATS conference also will include results from respiratory imaging (medical imaging of the lungs) that were obtained from the trial.
Further testing will be done in a Phase 2b trial, to pave the way for a critical Phase 3 trial.
“We are pleased to present this study at ATS, which builds on our current understanding of pulsed iNO therapy to improve pulmonary hemodynamics and exercise capacity in PH-IPF patients. The next step in this program will be to conduct a larger, controlled Phase 2b study to inform the design of a pivotal Phase 3 trial,” said Fabian Tenenbaum, CEO of Bellerophon Therapeutics.
Bellerophon also is testing the INOpulse device in patients with PH-chronic obstructive pulmonary disease (PH-COPD) and pulmonary arterial hypertension (PAH) in clinical trials.

One comment