The study of metastasis — the spread of cancer cells from one organ to another — has historically been bereft of good models as cancer researchers struggle to more fully understand the process of tumor progression fully, with new therapies targeting main causes of cancer-related death being painfully slow in development.
Consequently, news that researchers at Houston Methodist have invented a new, ex vivo lung cancer model that mimics the tumor progression process, is an advancement that is most welcome to the scientific community.
A paper reporting results of tests conducted on the model are published this month in the journal The Annals of Thoracic Surgery entitled “Ex Vivo Four-Dimensional Lung Cancer Model Mimics Metastasis“ (The Annals of Thoracic Surgery April 2015, Vol.99(4):11491156, doi:10.1016 j.athoracsur.2014.08.085). The paper is coauthored by Dhruva K. Mishra, PhD of the Houston Methodist Research Institute Department of Surgery; Yiqun Zhang, MA, and Fengju Chen, MS, of the Dan L. Duncan Cancer Center Division of Biostatistics in Houston; Chad J. Creighton, PhD, of the Baylor College of Medicine at Houston; Michael J. Thrall, MD, of the Houston Methodist Hospital Department of Pathology and Genomic Medicine; and lead author Min P. Kim, MD of the Weill Cornell Medical College at Houston Methodist Hospital Department of Surgery
The coauthors note: “We have developed a four-dimensional (4D) lung cancer model that forms perfusable tumor nodules. We determined if the model could be modified to mimic metastasis. We modified the 4D lung cancer model by seeding H1299, A549, or H460 cells through the trachea only to the left lobes of the acellular lung matrix. The model was modified so that the tumor cells can reach the right lobes of the acellular lung matrix only through the pulmonary artery as circulating tumor cells (CTC). We determined the gene expressions of the primary tumor, CTCs, and metastatic lesions using the Human OneArray chip.”
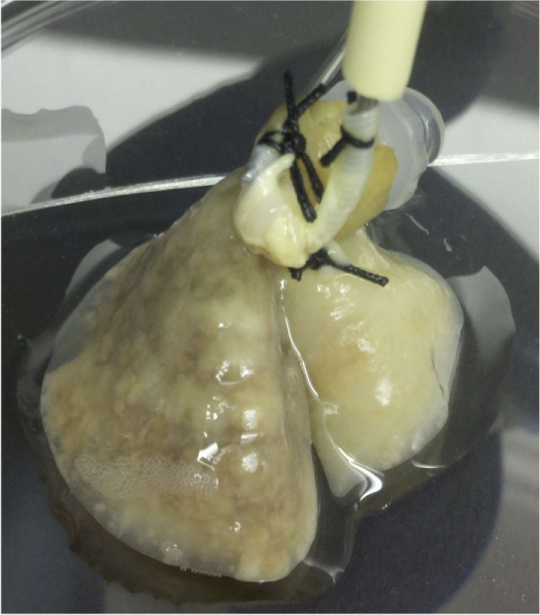
The researchers conclude that their 4D lung cancer model can isolate tumor cells in three phases of tumor progression, and that this model may mimic the biology of lung cancer metastasis and may be used to determine its mechanism and potential therapy in the future. The model can also be employed to study progression of other types of cancer than lung.
[adrotate group=”10″]
 “Our model truly captures the phenomenon of cancer metastasis,” comments Houston Methodist thoracic surgeon and scientist, Min P. Kim, M.D., the report’s principal investigator.
“Our model truly captures the phenomenon of cancer metastasis,” comments Houston Methodist thoracic surgeon and scientist, Min P. Kim, M.D., the report’s principal investigator.
The “4-D” model is created by removing all the cells from a vertebrate lung, leaving the enveloping matrix to provide cell growth and development support. Once cells are removed, the native lung matrix is further modified, then placed in a bioreactor to nurture human tumor cell growth.
Dr. Kim explains that unlike other tumor models, the 4-D model allows tumor cells to form 3-D nodules that grow over time. Dr. Kim calls an earlier version of the model “3-D ex vivo,” and the latest iteration of the model’s fourth dimension is flow, incorporating movement of fluids between the lungs through blood vessels.
This fourth dimension allows the model to reveal the growth of primary tumors, the formation of CTCs and formation of metastatic lesions — three steps of cancer progression that are not addressed in any previous single in vitro or ex vivo model. Also unlike foregoing in vivo models of metastasis, which often require researchers to wait months for useful information about metastasis progression, the 4-D model can provide usable data within a matter of days.
In their study, Dr. Kim and his colleagues also investigated gene expression in cancer cells during different phases of tumor progression, finding gene signatures of experimental CTCs to be associated with poor lung cancer patient survival.
“The model allowed for the isolation of unique gene signature of circulating tumor cell phase of metastasis, which may provide a clue to the mechanism of tumor progression,” Dr. Kim observes in a Houston Methodist release.
In future experiments, Dr. Kim says his group will be focusing on circulating tumor cells’ unique gene signatures in order to better understand mechanisms of tumor progression, noting that this has potential to generate new ideas for therapies that could stop metastatic spread in lung cancer patients in its tracks.
The Annals of Thoracic Surgery paper’s coauthors received funding support from the American Association for Thoracic Surgery Graham Research Foundation, the Houston Methodist Foundation, the National Institutes of Health, and the Cancer Research & Prevention Institute of Texas.
Sources:
Houston Methodist Research Institute
The Annals of Thoracic Surgery
Image Credits:
Dr. Min Kim Lab
Houston Methodist Research Institute