In a new study entitled, “Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomized, double-blind, placebo-controlled trial,” researchers demonstrate that Alpha1-Proteinase Inhibitor therapy decreases progression of lung tissue loss in patients with alpha-1 antitrypsin deficiency (AATD). The results were obtained from the largest clinical trial (The RAPID study) performed in AATD patients by CSL Behring, a leader in the plasma protein therapeutics industry. The study was published in the journal The Lancet.
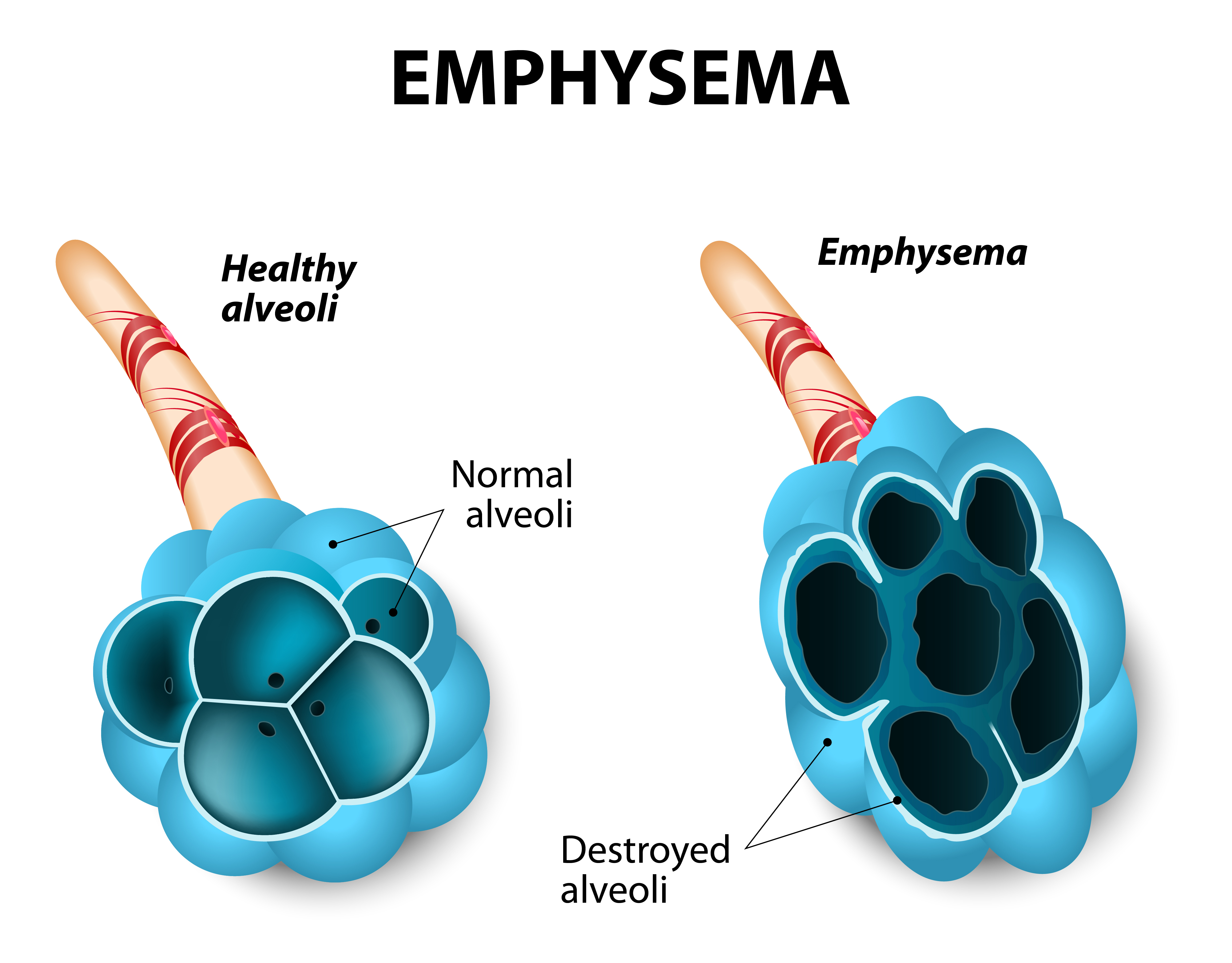
Alpha-1 antitrypsin deficiency (AATD) is characterized by the lack or very low levels of a protein — alpha-1-proteinase inhibitor (A1-PI) — whose function is to inhibit the enzyme neutrophil elastase. This enzyme is released by neutrophils and macrophages to fight infections, however, if uncontrolled can induce damage in host tissue (particularly in the lungs) in the absence of its inhibitor alpha-1 antitrypsin. AATD is an inherited disorder and severe loss of A1-PI increases patients’ risk of developing emphysema, a form of chronic obstructive pulmonary disease (COPD).
In the current study, researchers performed the first randomized, placebo-controlled trial to assess the benefits of treating AATD patients with A1PI therapy. The team recruited AATD patients from 28 international study centers in 13 countries and measured the effect of Alpha1-Proteinase Inhibitor therapy on the progression of emphysema by chest computed tomography (CT) lung scan for two years when compared to patients in a placebo-control group. Patients’ outcomes were determined by measuring the rate of lung density loss at total lung capacity (TLC) combined with patients’ functional residual capacity (FRC), a measure that indicates the volume of gas remaining in the lungs at the end of expiration. Both TLC and FRC were measured separately at 0, 3, 12, 21, and 24 months.
The authors found that while there were no significant differences in the annual rate of lung density loss at TLC and FRC combined between A1PI and placebo-control patients, when measured at TLC alone they observed that the A1PI group of patients had a much lower annual rate of lung density loss when compared to the placebo-control group (specifically, a 34 percent reduction). However, this difference was lost when measuring the FRC alone.
This means that in fact A1PI therapy slows progression of emphysema, however, this phenotype was only confirmed by measuring lung density with CT lung scan at TLC alone. Assessing patients’ therapy outcomes by lung density measurement at FRC alone or by combining both measures, TLC and FRC, failed to detect the positive benefits on AATD patients’ lungs. In light of their results, the team suggests that enhancing A1PI therapy should be considered as a measure for preserving lung function in patients with emphysema, due to severe α1 AATD.
Kenneth R. Chapman, MD, Director of the Asthma & Airway Centre at the University Health Network in Toronto and study lead author commented, “RAPID is regarded as a landmark study validating almost two decades of focus on the lung-density endpoint as the most sensitive way to track lung tissue decline and the seven-year collaboration of an international team of investigators. Our findings provide additional evidence that treatment with an Alpha1-Proteinase Inhibitor may slow the accelerated loss of lung tissue that is a characteristic of this potentially debilitating disease.”
“We are excited that the results of this important study in Alpha-1 have been published in the highly-respected journal The Lancet. We commend CSL Behring for their outstanding commitment to the Alpha-1 community and advancing the understanding and treatment of the disease. These results further support the use of augmentation therapy in the treatment of Alpha-1, and we hope they bolster efforts of Alpha-1 communities around the world to win access to therapy,” added John Walsh, co-founder, President and CEO of the Alpha-1 Foundation.

No matter how much news. Be it good on augmentation therapy or any other costly meds we Alpha’S living in the UK may as well be on another planet. Although the illness was given its name in the early 60s it is still classed as a rare disease in this country. We have a handful of doctors throughout the UK that know a little on A1AD there is hardly any training whatsoever for junior doc’s or nurses in the NHS and NICE find it hard to fund any medication for this illness. Which is why we are put under the umberella of COPD which most Alphas know to be a joke. We need augmentation therapy if we are going to be given a chance of living a decent lifetime.