In the course of a new study at the Harvard T.H. Chan School of Public Health in Boston, scientists have made an unexpected discovery suggesting intriguing new avenues both for basic biological research and for therapeutic interventions to fight asthma.
Karen Feldscher of Harvard Chan School Communications reports that in people with asthma, cells lining airways in the lungs are unusually shaped, and “unscramble around like theres a fire drill going on.” However, the new findings at HCSC could also be important new knowledge for research in other areas, notably cancer, where similar types of cells play a major role.
“Until now, scientists thought that epithelial cells which line not only the lungs airways but major cavities of the body and most organs just sat there motionless, like tiles covering a floor or cars jammed in traffic,” Jeffrey Fredberg, a professor of bioengineering and physiology at the Harvard Chan School and one of the senior authors of a study paper published online Aug. 3 in the journal Nature Materials told Ms. Feldscher. “But the study showed that, in asthma, the opposite is true.”
The Nature Materials article, entitled “Unjamming . cell shape in the asthmatic airway epithelium” (Nature Materials (2015) dpi:10.1038/nmat4357)” is coauthored by Jin-Ah Park, Jae Hun Kim, Jennifer A. Mitchel, Nader Taheri Qazvini, Chan Young Park, Maureen McGill, Sae-Hoon Kim, Bomi Gweon, Jacob Notbohm, Robert Steward Jr, Stephanie Burger, Dhananjay T. Tambe, Corey Hardin, Stephanie A. Shore, James P. Butler, Jeffrey M. Drazen, and Jeffrey J. Fredberg of the Harvard T.H. Chan School of Public Health, Boston, Massachusetts; Dapeng Bi and M. Lisa Manning of Syracuse University, Syracuse, New York; Nader Taheri Qazvini of the School of Chemistry, College of Science, University of Tehran, Iran; Kelan Tantisira, Elliot Israel, Elizabeth P. Henske, Scott T. Weiss, and James P. Butler of Brigham and Womens Hospital, Harvard Medical School, Boston; Scott H. Randell of The University of North Carolina at Chapel Hill; Alvin T. Kho of Children’s Hospital, Boston; Dhananjay T. Tambe of the Department of Mechanical Engineering at the University of South Alabama, Mobile, Alabama; David A. Weitz of Harvard University, Cambridge, Massachusetts; and Daniel J. Tschumperlin of the Mayo Clinic College of Medicine at Rochester, Minnesota.
The coauthors note that materials ranging from coffee beans flowing in a chute to cells remodeling in a living tissue, a wide variety of close-packed collective systems — both inert and living — have the potential to jam. This eclectic collective of substances can sometimes flow like a fluid or jam and rigidify like a solid.
Observing that the unjammed-to-jammed transition remains poorly understood, and structural properties characterizing these phases remain unknown, using primary human bronchial epithelial cells, the scientists show in their paper that the jamming transition in asthma — a disorder that afflicts more than 300 million people worldwide — is linked to cell shape, thus establishing in that system a structural criterion for cell jamming, and that surprisingly, the collapse of critical scaling predicts a counter-intuitive relationship between jamming, cell shape and cell-to-cell adhesive stresses borne out by direct experimental observations.
The researchers determined that cell shape thus provides a rigorous structural signature for classification and investigation of bronchial epithelial layer jamming in asthma, and potentially in any process in disease or development in which epithelial dynamics play a prominent role.
The study included lead authors Jin-Ah Park and Jae Hun Kim, research scientists in the HCSC Department of Environmental Health who study asthma, and Jeffrey M. Drazen, a pulmonologist and professor in the department, who studies mechanotransduction in asthma — how the bronchial constriction of asthma might trigger cell changes in the epithelium. The study also included mathematical physicists James Butler, a senior lecturer on physiology in the Department of Environmental Health, and M. Lisa Manning and Max Bi at Syracuse University, as well as colleagues from the Harvard Chan School and other Harvard institutions.
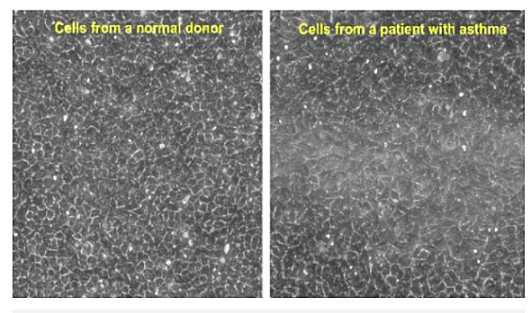 Photo caption: Asthma cells on the move. Scientists thought epithelial cells just sat there motionless, like tiles. A new Harvard study is shaking things up. Images courtesy of Jeffrey Fredberg and Jin-Ah Park
Photo caption: Asthma cells on the move. Scientists thought epithelial cells just sat there motionless, like tiles. A new Harvard study is shaking things up. Images courtesy of Jeffrey Fredberg and Jin-Ah Park
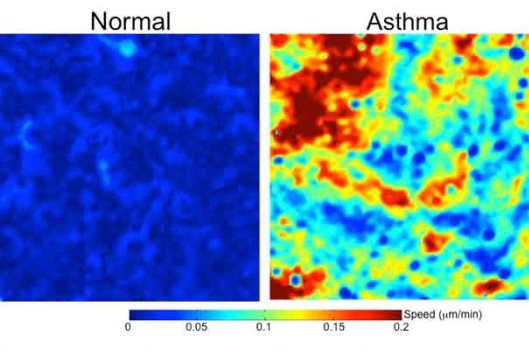
In her report, Ms. Feldscher notes that in order to analyze cell movement, the researchers took time-lapse images of epithelial cells, and also produced videos that quite vividly show differences between normal and asthmatic cells, with the normal cells being nearly pentagon-shaped and jammed so they can hardly move at all while the asthmatic cells become more spindle-shaped and constantly move and swirl without jamming.
In order to analyze the mechanical forces in play, the researchers placed layers of epithelial cells from either normal airways or asthmatic airways on a soft gel surface simulating the stiffness of the lung. As the cells moved, their push-pull motion caused the gel to move as well, enabling the researchers to deduce the mechanical forces at work among the cells.
“During maturation, cells undergo a phase transition from an unjammed state, in which cells move like a fluid and rearrange with neighbors frequently, to a jammed state, in which cells move little like a solid and rearrange with neighbors infrequently,” notes co-lead author of the study Jin-Ah Park. “As compared with normal cells, this phase transition toward the jammed state is delayed in asthmatic cells. Perturbation of jammed cells with compressive mechanical stress reverses the natural and spontaneous phase transition, causing the jammed cells to revert to an unjammed state. In the same publication, we also reported that cell-shape parameter can be a rigorous tool in determining whether cells are in a jammed or an unjammed state. My research investigating the physical properties of the asthmatic airway epithelium can be a foundation for a better understanding of the impaired airway epithelium in asthma.” The Park lab site is here.
Having determined that epithelial cells in asthmatics’ airways are oddly shaped and not jammed, the scientists’ next order of business is to figure out why this is happening — i.e.: is it the asthma causes the cells to unjam, or does the unjamming of the cells cause the asthma?
“It’s a very big question to figure out why this particular cell shape and movement is happening,” says Jin-Ah Park. “We know that asthma is related to genes, environment, and the interaction between the two, but asthma remains poorly understood.”
“Whatever the reason, knowing more about how these cells jam and unjam is important,” Dr. Fredberg tells Ms. Feldscher, “because epithelial cells play a prominent role not just in asthma, but in all processes involving cell growth and movement, including organ development, wound healing, and, importantly, cancer. The findings open the door to new possibilities for developing drugs to fight asthma as well as other diseases and to new research questions.”
“Trying to define how cells behave, how they exert forces on each other, and how that changes what they do are big open questions,” continues Dr. Fredberg. “Researchers all over the world are looking more and more at these questions. Its very exciting.”
Sources:
Harvard T.H. Chan School of Public Health
Nature Materials
Image Credits:
Harvard T.H. Chan School of Public Health
Jeffrey Fredberg and Jin-Ah Park