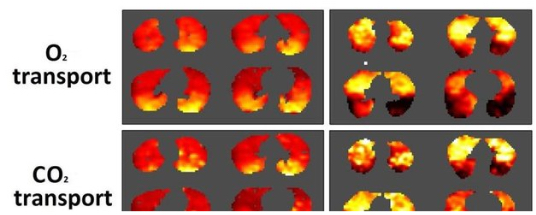
Researchers at Aarhus University in Aarhus, Denmark, have for the first time succeeded in producing 3-D images showing oxygen and carbon dioxide (CO2) transport in the lungs. This new technique provides hope for better treatment of diseases like chronic obstructive pulmonary disease (COPD) and lung cancer.
With every breath we take, oxygen and CO2 are transferred between our blood and lungs — a process crucial to maintaining respiratory system health and life. More detailed knowledge about the movement of oxygen and CO2 is particularly important to patients with pulmonary lung diseases such as COPD, lung cancer, and asthma, and also for acutely ill patients on respirators, for whom each new research finding may turn out to be the first step on the road to more effective forms of treatment.
“We are the first to develop a new model for how you can see into the lungs. The model provides a kind of 3D map of how and where the CO2 and oxygen transfers take place,” says engineer and PhD student Troels Johansen from the Department of Clinical Medicine at Aarhus University.
Working in collaboration with researchers from Harvard Medical School, Troels Johansen has developed a mathematical model as part of his PhD project that provides the basis for the 3-D images, which in turn are developed from PET scans. This new method was recently published online, before its print edition, in the journal Respiratory Physiology & Neurobiology.
The paper, “A method for mapping regional oxygen and CO2 transfer in the lung“ (Respiratory Physiology & Neurobiology Volume 222, 1 February 2016, Pages 2947 doi:10.1016/j.resp.2015.10.017), presents a method to map pulmonary O2 and CO2 transfer rates in three dimensions, sensitivity analyses of errors in imaging data and in global variables, and quantitative three-dimensional maps of O2 and CO2 transfer rates that may yield valuable insights into lung function in health and disease. These maps describe the contribution of anatomical regions to overall gas exchange and demonstrate how transfer rates of the two gas species differ regionally.
An algorithm for generating such maps is presented, as are regional gas transfer maps using values of ventilation and perfusion imaged by PET/CT contrasting gas transfer in, for example, a healthy subject paired with an asthmatic patient after bronchoprovocation.
The co-authors observe that gas transfer maps offer an intuitive display of physiologically relevant lung function at a regional level, and may improve understanding of pulmonary gas exchange and serve as a pre-surgical evaluation tool.
“For example, if we take cancer patients with a tumor in the lung, it will be easier to predict the consequences of removing part of the lung by surgery,” says Johansen in a release. “It will also be easier for doctors to determine the COPD patients who will benefit from an operation and those who will not. We also believe that the new model will come to contribute with knowledge that can help patients in intensive care who are on a respirator. The new model is not only able to make it easier for doctors to foresee the consequences of high-risk lung operations. It will also contribute new basic knowledge about the crucial oxygen and CO2 transfer in both healthy and diseased lungs.”
Aarhus University, founded in 1928, is a modern research-intensive university, the country’s second-oldest and its largest after a merger with the Aarhus School of Engineering. With nationwide and international clout across the entire research spectrum, and placing a high priority on maintaining close connection to business and industry, the university takes pride in its engagement with the development of the society to which it belongs. Over the last decade, Aarhus has consolidated its position in the top 100 of the most influential university rankings.
Sources:
Aarhus University
Respiratory Physiology & Neurobiology