 Amsterdam-based Royal Philips and the Toronto, Canada advocacy and funding organization Grand Challenges Canada (GCC) are collaborating on an innovative project to improve childhood
Amsterdam-based Royal Philips and the Toronto, Canada advocacy and funding organization Grand Challenges Canada (GCC) are collaborating on an innovative project to improve childhood  pneumonia diagnoses in low-resource countries. The announcement was made at the fourth annual Women Deliver Conference 2016 in Copenhagen, Denmark, last month.
pneumonia diagnoses in low-resource countries. The announcement was made at the fourth annual Women Deliver Conference 2016 in Copenhagen, Denmark, last month.
Funded by the Canadian government, GCC is dedicated to supporting bold ideas designed to have a big impact in global health. The organization funds innovators in low- and middle-income countries (LMICs) and in Canada, and promotes integration of science and technology with social and business innovation in a synergy GCC calls “integrated innovation,” focusing on scaling successful innovation with an emphasis on results — saving and improving lives.
GCC works closely with Canada’s International Development Research Centre
(IDRC), the Canadian Institutes of Health Research (CIHR), and Global Affairs Canada,
to increase scale, sustainability, and impact.
Royal Philips is a global health technology company focused on improving people’s health and enabling better outcomes across the health continuum from healthy living and prevention to diagnosis, treatment, and home care. Philips leverages its advanced technology and deep clinical and consumer insights to deliver integrated solutions — particularly in the fields of diagnostic imaging, image-guided therapy, patient monitoring, and health informatics.
The Philips and GCC partnership is a repayable grant agreement for the manufacturing and distribution of the Philips Children’s Respiration Monitor (ChARM) device, making it affordable and accessible for community-based health workers in low-income settings throughout the world.
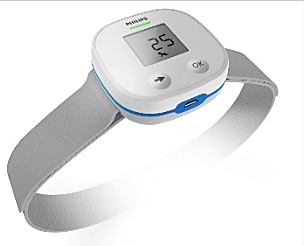 The ChARM unit converts chest movements detected by accelerometers into an accurate breathing count, using specially developed algorithms. It not only provides quantitative feedback, but also qualitative feedback to the healthcare provider based on the World Health Organization’s IMCI 3 (Integrated Management of Childhood Illness) guidelines to diagnose fast breathing rates — one of the key vital signs for diagnosing pneumonia.
The ChARM unit converts chest movements detected by accelerometers into an accurate breathing count, using specially developed algorithms. It not only provides quantitative feedback, but also qualitative feedback to the healthcare provider based on the World Health Organization’s IMCI 3 (Integrated Management of Childhood Illness) guidelines to diagnose fast breathing rates — one of the key vital signs for diagnosing pneumonia.
Philips says ChARM has the potential to assist community health workers in establishing a more accurate measurement of a sick child’s breathing rate to help improve pneumonia diagnosis and potentially prevent some of the 922,000 childhood deaths caused by the respiratory disorder each year.
According to a WHO fact sheet, pneumonia remains the leading infectious cause of death around the world in children younger the 5, killing nearly 2,500 children a day, with most victims younger than 2 years old. Most of these deaths are in poor areas in developing countries where treatment for children is not readily available. UNICEF and the WHO have made pneumonia one of their prime focus areas in efforts to reduce child mortality.
 “As a leading health technology company, Philips’ vision is to improve people’s lives through meaningful innovation,” Dr. Maarten van Herpen said in a press release. He is head of the Philips Africa Innovation Hub, which will serve as the center for developing innovations in Africa-for-Africa in areas of healthcare, lighting, and healthy living.
“As a leading health technology company, Philips’ vision is to improve people’s lives through meaningful innovation,” Dr. Maarten van Herpen said in a press release. He is head of the Philips Africa Innovation Hub, which will serve as the center for developing innovations in Africa-for-Africa in areas of healthcare, lighting, and healthy living.
“Equitable innovation strategies can help drive sustainable solutions that bridge the divide between the privileged and lesser privileged sections of society, to improve the quality of life for all,” he said. “Thanks to collaborations and co-investments like the one we have signed with Grand Challenges Canada, companies such as Philips can scale innovations that reach an underserved population and thereby integrate the United Nations Sustainable Development Goal 3 — ensure healthy lives and promote well-being for all at all ages, into their core business strategies.”
Development of the ChARM device is part of the commitment made in Philips’ pledge to the Every Woman Every Child movement — a global initiative spearheaded by the U.N. secretary general to improve the health of women and children that mobilizes international and national action by governments, the U.N., multilaterals, the private sector, and civil society, to address major  health challenges. The Every Woman Every Child movement provides a structure for putting into action the Global Strategy for Women’s, Children’s and Adolescent’s Health, which proposed a road map for ending preventable deaths of this group within a generation.
health challenges. The Every Woman Every Child movement provides a structure for putting into action the Global Strategy for Women’s, Children’s and Adolescent’s Health, which proposed a road map for ending preventable deaths of this group within a generation.
Philips says its mission is to improve the lives of billions of people through delivering innovations that help make life healthier and more sustainable through technologies that can help improve the lives of mothers and children around the world. To further the goals of Every Woman Every Child, Philips will target populations of women and children where maternal and child mortality are concentrated, and where nutrition and energy challenges are most acute, with a special focus on sub-Saharan Africa
and Southeast Asia.
Principal goals of the Pledge are:
- Deliver the next generation of care for mothers and their children right from the start;
- Provide better access to healthcare by creating solutions which enable healthier, safer living;
- Promote healthy and nutritious diets for mothers and children, understanding barriers to breastfeeding and supporting breastfeeding in the workplace.
Promotion and scaling of such innovations is important in order to accelerate implementation of the U.N. Global Strategy for Women’s, Children’s, and Adolescents’ Health in support of the Sustainable Development Goals.
GCC is collaborating with Philips to invest in ChARM in response to the global call for innovation to prevent and treat pneumonia. GCC’s CA$602,000 repayable grant is being matched by Philips to finance the market launch of ChARM, and support development of the device’s next generation, which is planned to include pulse oximetry. The agreement also aims to ensure access to the device in low- and middle-income countries.
 “Pneumonia is a killer of children, especially in the developing world. ChARM responds to a crucial need to reduce deaths from childhood pneumonia,” said GCC CEO Dr. Peter A. Singer. “By collaborating with a large company such as Philips, we can help innovations to reach millions.”
“Pneumonia is a killer of children, especially in the developing world. ChARM responds to a crucial need to reduce deaths from childhood pneumonia,” said GCC CEO Dr. Peter A. Singer. “By collaborating with a large company such as Philips, we can help innovations to reach millions.”
Based on estimated projections by Philips, ChARM has a significant potential to save and improve lives, enabling more than 100 million children per year to receive more accurate pneumonia diagnosis support.
Philips said an important aspect in diagnosing pneumonia is monitoring a child’s breathing rate. In the developing world, community health workers typically count how many breaths a child takes in the span of a minute to detect and diagnose the condition, but achieving an accurate count using that method can be tricky since shallow breaths are difficult to detect, children tend to move around, and there are frequently distractions and other checks to perform.

To overcome these challenges, Philips ChARM can be strapped around a child’s chest (direct skin contact is unnecessary) and delivers reliable breathing measurements by converting chest movements detected by accelerometers into an accurate breath count. The ChARM device will be dustproof, water-splash proof, and accurate in extreme temperatures. A long-lasting battery will make ChARM power-independent, suitable for use in locations without electricity. The device is small and easily portable, with a pictogram-based user interface designed to be intuitive, easy to use, and suitable for operators with low literacy.
Field testing of the Philips ChARM has been conducted in East Africa and India, leading to improvements in design and technology that have been incorporated on the basis of feedback from community health workers and clinical officers in low-resource settings.
The Philips Children’s Respiration Monitor is pending CE-marking and is expected to become commercially available later this year.

