Researchers from Germany have identified how Ofev (nintedanib) and Esbriet (pirfenidone) work — two FDA-approved drugs for the treatment of idiopathic pulmonary fibrosis (IPF).
The study, titled “A Novel Antifibrotic Mechanism Of Nintedanib And Pirfenidone: Inhibition Of Collagen Fibril Assembly,” was published in the American Journal of Respiratory Cell and Molecular Biology.
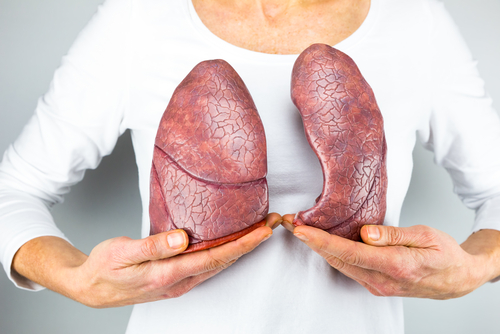
Lung fibrosis, or scarring, results from the accumulation of fibrotic cells due to the increased production of collagen. This leads to scarring of the lung tissue and affects oxygen uptake, reducing tissue elasticity and causing a decline in respiratory function.
“Currently, the drugs nintedanib [Ofev] and pirfenidone [Esbriet] are widely used in treatment,” Claudia Staab-Weijnitz, senior author of the study, from the Institute of Lung Biology at Helmholtz Zentrum München, said in a news release. Both drugs slowed the progression of the disease, but how they worked was not completely understood, she said.
Researchers treated human cells from IPF patients and healthy donors with Ofev and Esbriet in the absence or presence of TGF-β1, a protein involved in fibrosis.
Results showed that both IPF drugs reduced the expression levels of several proteins that participate in the process of fibrosis, and decreased the accumulation of collagen fibrils in IPF cells compared to those of healthy donors. Both drugs acted in a dose-dependent manner.
“Our analyses showed that both [Ofev] and [Esbriet] inhibit the formation of collagen fibrils,” Staab-Weijnitz said. “Specifically, they reduce the formation of new collagen molecules and secondly, prevent them from forming into larger assemblies, so-called fibrils.”
Ofev was more effective in reducing the expression of genes that promote fibrosis and collagen production, but both drugs inhibited fibril formation.
Together, these findings, based on human cells, revealed a novel mechanism of action for both Ofev and Esbriet.
Researchers now plan to further look into the mechanisms of fibril formation and study methods to halt fibrosis in patients with lung disease.
“The results show that the optimized human system allows the study of collagen biosynthesis and collagen fibril formation at all regulatory levels,” Staab-Weijnitz said. “Thus, it is very well suited for use as an initial test system for novel therapeutic strategies for lung fibrosis.”

Early evidence of efficacy of PBI-4050 alone and also in combination with one of the commercially available IPF drugs
PBI-4050 shown to be very well tolerated whether used alone or in combination with nintedanib or pirfenidone
LAVAL, QC, Nov. 17, 2016 /CNW Telbec/ – ProMetic Life Sciences Inc. (TSX: PLI) (OTCQX: PFSCF) (“ProMetic” or the “Corporation”) announced today positive interim results from its Open Label Phase 2 clinical trial in patients suffering from idiopathic pulmonary fibrosis (“IPF”). In addition to demonstrating that PBI-4050 is safe and well tolerated in patients suffering from IPF, the objective of this study was to provide early evidence of clinical benefits of PBI-4050 treatment whether used alone or in addition to either nintedanib or pirfenidone. Forty patients are enrolled in the study in 6 sites across Canada. At this time, the Corporation is reporting on the first 30 patients that have completed their 12 weeks of treatment.
“We are very pleased with the results to date from this study as they provide us with very important data points relating to efficacy and safety” stated Dr. John Moran, Chief Medical Officer of ProMetic. “There is early evidence of efficacy in the patients treated with PBI-4050 alone and in those treated with PBI-4050 in combination with one of the commercially available drugs. This compares favourably to that of the pirfenidone or nintedanib treatment reported in the ASCEND trial and in the two INPULSIS trials, respectively. Further good news is the fact that PBI-4050 is very well tolerated by IPF patients whether used alone or in combination with either nintedanib or pirfenidone” added Dr. Moran.
Very good news.
Help me how to heal the scars in my lungs
I was on Ofev & did not tolerate it well at all. Arthritis blew up in hands like crazy & diarrhea got too bad, along with headaches, nausea, etc. I was told later that I did not have IPF, but too many other factors to bare.
I was told Ofev stops 3 of the scarring reflex/growth factors of the 7 known ones. I always wondered if Esbriet worked on the others? Denise
Denise,
I too am on ofev 100mg and was unable to tolerate the higher dose. In Feb 2017 , arthritis set in real fast in hands and wrists, unable to even shake hands. Thus arthritis treatment set in which in turn shut down the immune system, consequently, I am just recovering from a bout with pneumonia. And, I have put all the meds on hold for now.
Thus I am interested in what was your prescribed path after the arthritis. Are you back on Ofev?
Please let me know .
Regards
George F.
Has anybody tried these drugs on post lung transplant patients to prevent chronic rejection?