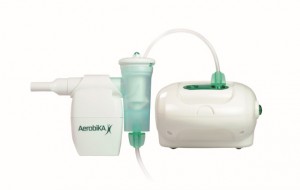
 A recent study conducted at the Robarts Research Institute at Western University in London, UK, demonstrated the effectiveness of the drug-free Aerobika device for the treatment of both chronic obstructive pulmonary disease (COPD) and bronchiectasis after three weeks of use every day. The device was developed and commercialized by Monaghan Medical Corporation and is used by many leading hospitals in the United States.
A recent study conducted at the Robarts Research Institute at Western University in London, UK, demonstrated the effectiveness of the drug-free Aerobika device for the treatment of both chronic obstructive pulmonary disease (COPD) and bronchiectasis after three weeks of use every day. The device was developed and commercialized by Monaghan Medical Corporation and is used by many leading hospitals in the United States.
The study demonstrated that patients using Aerobika daily for three weeks increased mucus clearance, decreased cough frequency and breathlessness, and enhanced exercise tolerance. In addition, the scientists also discovered that the device induces an overall increase in the quality of life without side effects, as reported by the patients themselves who tested the device.
“The results from the Robarts study provide compelling evidence that patients suffering from COPD, Cystic Fibrosis (CF) or Bronchiectasis can now have an improved quality of life and a safe and easy-to-use method to address the unmet need of mucus clearance and potentially reduce the need for hospitalization,” said the Vice President of Clinical Affairs at Monaghan Medical Corporation, Dominic Coppolo.
The researchers assessed Aerobika’s effectiveness through the Pulmonary Function Test, Six Minute Walk Test, the St. George’s Respiratory Questionnaire (SGRQ), the Patient Evaluation Questionnaire (PEQ), and Hyperpolarized Helium-3 Magnetic Resonance Lung Imaging (3He MRI).
[adrotate group=6″]
The results of the study corroborate a different study conducted by Trudell Medical International (TMI), which revealed the safety and effectiveness of Aerobika for the treatment of COPD, as previously reported by Lung Disease News. The findings of the study were recently presented at the 2014 European Respiratory Society (ERS) Conference in Munich, Germany.
The Aerobika Oscillating Positive Expiratory Pressure (OPEP) device is a treatment that does not require any drugs, but uses a proprietary pressure-oscillation dynamic, which is able to offer intermittent resistance, as well as positive pressure and oscillations at the same time. With these movements and pressure, the device releases and clears mucus, unclogging the airways. The device was recently granted the Gold medal at the Medical Design Excellence Awards (MDEA), a contest that recognizes innovative medical devices that are able to improve patients’ care and quality of life.
COPD is characterized by excessive mucus, which causes difficulty in breathing. When the mucus clogs the lungs, it can cause bacteria and infections, and in severe cases exacerbations, hospitalizations, and even death. The disease affects over 24 million people in the United States alone, costing the country $50 billion per year in medical expenses and lost productivity.


I just started using the Aerobika flutter valve first day my throat was sore and felt somewhat tight. Today when I used it, I had phlegm in my throat and made it feel tighter and more horse. Does the flutter bother the muscles in the throat or voice box? I really want to use this cos I think it will help but don’t want my throat to swell! If you or anyone knows please let me know asap… thank you so much… Janet