Tustin, California based Medical biotechnology company Uptake Medical reports that clinicians have successfully performed the first-ever regional lung cancer tumor ablation using Uptake’s InterVapor Targeted Vapor Therapy system.
A patient diagnosed with colorectal cancer with metastasis to the lung underwent the procedure, which was performed by Prof. Arschang Valipour, an interventional pulmonologist at The Otto-Wagner-Spital Hospital in Vienna, Austria. Prof Valipour is a lead clinical investigator in a new treat-and-resect clinical trial evaluating InterVapor’s potential applicability to treat patients with early stage lung cancer and lung metastases.
InterVapor Therapy is an innovative approach to treating emphysema and lung cancer — two leading causes of mortality and disability worldwide. InterVapor provides a non-surgical option for treatment, using a standard bronchoscope to deliver a precise stream of heated water vapor through the lungs’ pathways to the afflicted area. The powerful energy of the vapor provides controlled, rapid ablation or physical removal of layers diseased lung tissue, leaving healthier sections unaffected. The outside-in bronchoscopic approach eliminates the need for percutaneous access or the need to pierce the tumor in a 10-second ablation during what is typically a 15-minute procedure
Ablation as a treatment for lung tumors is not new, but using water vapor as the ablation medium is. Heretofore, electronic probes have used to “burn” or “freeze”, and thereby remove cancerous tissue without resort to traditional surgery. Computed Tomography (CT), Ultrasound (US) or Magnetic Resonance Imaging (MRI) are variously used to assist the surgeon in guiding and positioning and inserting the needle probe at and into the tumor, at which point electrical energy is transmitted to the probe to “burn” or “freeze” the cancerous tissue.
According to Johns Hopkins Medicine in Baltimore, Maryland, “burning” in this context refers to increasing the temperature of the tumor to a level that causes cancer cells to die — usually achieved by radio frequency probes, referring to the type of energy used to increase the temperature, while “freezing” refers to cryoablation which decreases the temperature of the cancerous tissues to -40 C (-40 F) which also kills the cancer cells. Johns Hopkins says ablation’s effectiveness in treating cancer depends primarily on the size of the tumor and how accessible it is to the surgical probes, and that in general, for cancers three cm or less in size and easily accessible percutaneously, the aim is to completely kill the cancer. However, the larger the tumor the more difficult it is to achieve compete cancer death, making early treatment crucial if ablation is the chosen option. As well as lung cancer, ablation therapy can be employed for some liver cancer and kidney (renal) cancers as well.
Uptake’s InterVapor is the only vapor-based technology that enables targeted treatment of lung cancer and it has also received CE Mark approval allowing commercial sale of its InterVapor system in Europe to achieve bronchoscopic lung volume reduction in patients with heterogeneous upper lobe emphysema.
Advantages of InterVapor ablation therapy compared with conventional techniques are cited by Uptake Medical as including:
Non-Surgical
– InterVapor doesn’t involve surgery, implantation of devices, toxic drugs or chemicals, or lengthy procedures.
Highly Targeted
– Application of InterVapor Therapy’s a precise stream of heated water vapor can be localized to the more diseased areas of the lung, preserving healthier regions.
Easily Integrated
Applied using standard bronchoscopy, InterVapor can be easily incorporated in physicians treatment armamentarium.
Cost Effective
– InterVapor requires minimal infrastructure investment and is thus cost-effective across all parts of the health care system.
The InterVapor System has European CE Mark and Australian Therapeutic Goods Administration (TGA) approval, but does not yet have Food and Drug Administration (FDA) not available for sale in the U.S.A.
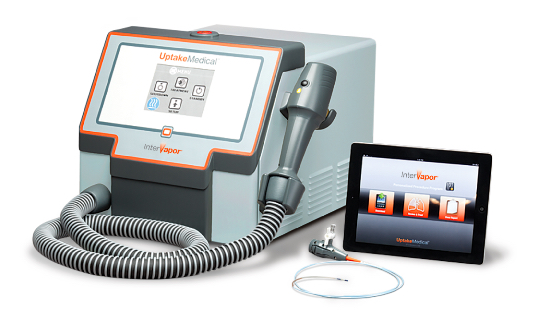
Uptake’s InterVapor System is comprised of three components: the InterVapor Personalized Procedure Program (IP3) tablet application, the InterVapor Catheter, and the InterVapor Generator.
The InterVapor Personalized Procedure Program (IP3) is a proprietary tablet-based application, which, based on a thorough review of each patient’s CT scan, recommends optimum treatment locations and ablation energy durations for each patient. This information is computed for each individual procedure, and securely delivered to the physician on a tablet computer. The physician chooses the vapor delivery location and duration at the time of the bronchoscopic treatment.
The InterVapor Generator is digitally controlled to precisely apply heated water vapor at specified levels, temperatures, and pressures, and the InterVapor Catheter is guided and positioned through a standard bronchoscope to deliver the prescribed vapor dose to the targeted lung segments. Each vapor delivery is computed by IP3 and lasts between 3-10 seconds.
 According to Prof. Dr. Med Felix Herth of ThoraxKlinik in Heidelberg, Germany, “Being granted the CE Mark validates for Uptake Medical the merits of our clinical hypothesis to have for the first time an option where we can treat the most diseased segments and preserve the less diseased segments.” Johns Hopkins Medicine notes that while ablation techniques may not be entirely successful for killing al of large or difficult to reach cancers, but having this procedure does not preclude patients from having other forms of treatment, and in such instances the patient can subsequently have any other treatment for which they are eligible, such as surgery, chemotherapy, or radiation.
According to Prof. Dr. Med Felix Herth of ThoraxKlinik in Heidelberg, Germany, “Being granted the CE Mark validates for Uptake Medical the merits of our clinical hypothesis to have for the first time an option where we can treat the most diseased segments and preserve the less diseased segments.” Johns Hopkins Medicine notes that while ablation techniques may not be entirely successful for killing al of large or difficult to reach cancers, but having this procedure does not preclude patients from having other forms of treatment, and in such instances the patient can subsequently have any other treatment for which they are eligible, such as surgery, chemotherapy, or radiation.
For the groundbreaking procedure he performed in Vienna, Prof. Valipour, using a unique, regional approach, accessed the patient’s left upper lobe sub-segment via a standard thin bronchoscope in order to place the InterVapor catheter in the airway. After confirming the catheter’s location with X-ray and ultrasound, he then delivered 8 seconds of InterVapor’s targeted vapor therapy to the region of the lung surrounding the tumor, the intention being to isolate and ultimately kill tumor cells while simultaneously preserving adjacent healthy tissue. The entire bronchoscopic procedure was completed in approximately 10 minutes, after which the patient underwent scheduled surgical resection to remove the area of the lung cancer tumor. Uptake Medical reports that histopathology analysis confirmed creation of a homogeneous ablation zone around the tumor following natural anatomical boundaries. Successful ablation of 90 percent of the tumor itself was completed, with complete tumor death projected within three days following the procedure.
“InterVapor is the only therapy with the promise of quick, simple, complete bronchoscopic ablation of lung tumor with margin.” Prof. Valipour comments in an Uptake release. “The vapor followed the patient’s anatomical boundaries and we successfully completed this first-in-human case without penetrating the tumor or puncturing adjacent healthy tissues, while creating a well-defined and controlled margin within the lung.”
According to the World Health Organization, lung cancer is the most common and deadliest cancer worldwide, accounting for 1.8 million new cases and 1.6 million deaths in 2012 (1), while the American Cancer Society reports that lung cancer claims more lives than colorectal, breast and prostate cancers combined. (2) Historically, patients have been treated only after becoming symptomatic, at which point advanced disease has usually taken hold with poor prognoses indicated, and only an estimated one-third of all lung cancer patients are considered candidates for conventional surgical treatment (3). However, Uptake maintains that as newer imaging and diagnostic technologies enable earlier lung cancer detection, demand for less-invasive interventions is increasing — particularly for patients with inoperable, multifocal, or recurrent lung cancer.
 “Current treatment options for lung cancer often require patients to undergo invasive surgery or tolerate toxic chemicals,” observes Uptake Medical’s president and CEO King Nelson. “We are very encouraged by the initial clinical results from InterVapor’s first-in-human case, and hope that regional tumor ablation performed with vapor will ultimately represent a new hope as a treatment option for the thousands of people worldwide who are battling lung cancer.”
“Current treatment options for lung cancer often require patients to undergo invasive surgery or tolerate toxic chemicals,” observes Uptake Medical’s president and CEO King Nelson. “We are very encouraged by the initial clinical results from InterVapor’s first-in-human case, and hope that regional tumor ablation performed with vapor will ultimately represent a new hope as a treatment option for the thousands of people worldwide who are battling lung cancer.”
The InterVapor platform is currently being tested and validated as a possible treatment for lung cancer tumors in a first-in-human study of regional lung tumor ablation in patients with early stage lung cancer and lung metastases. The regional bronchoscopic approach eliminates the need for percutaneous access or the need to pierce the tumor in a 10-second ablation during a 15-minute procedure.
Notes:
1) Fact Sheets by Cancer. World Health Organization. Web. 07 Aug. 2015. <http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=lung>.
2) Cancer Facts and Statistics 2015. American Cancer Society. Web. 07 Aug. 2015. <http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index>.
3) Alexander, ES & Dupuy DE, Lung Cancer Ablation: Technologies and Techniques, Semin Intervent Radiol 2013 Jun 30(2) 141-50. PubMed PMID: 24436530.
For more information, visit:
http://www.uptakemedical.com
Sources:
Uptake Medical Inc.
Johns Hopkins Medicine
Otto-Wagner-Spital Hospital
World Health Organization
American Cancer Society